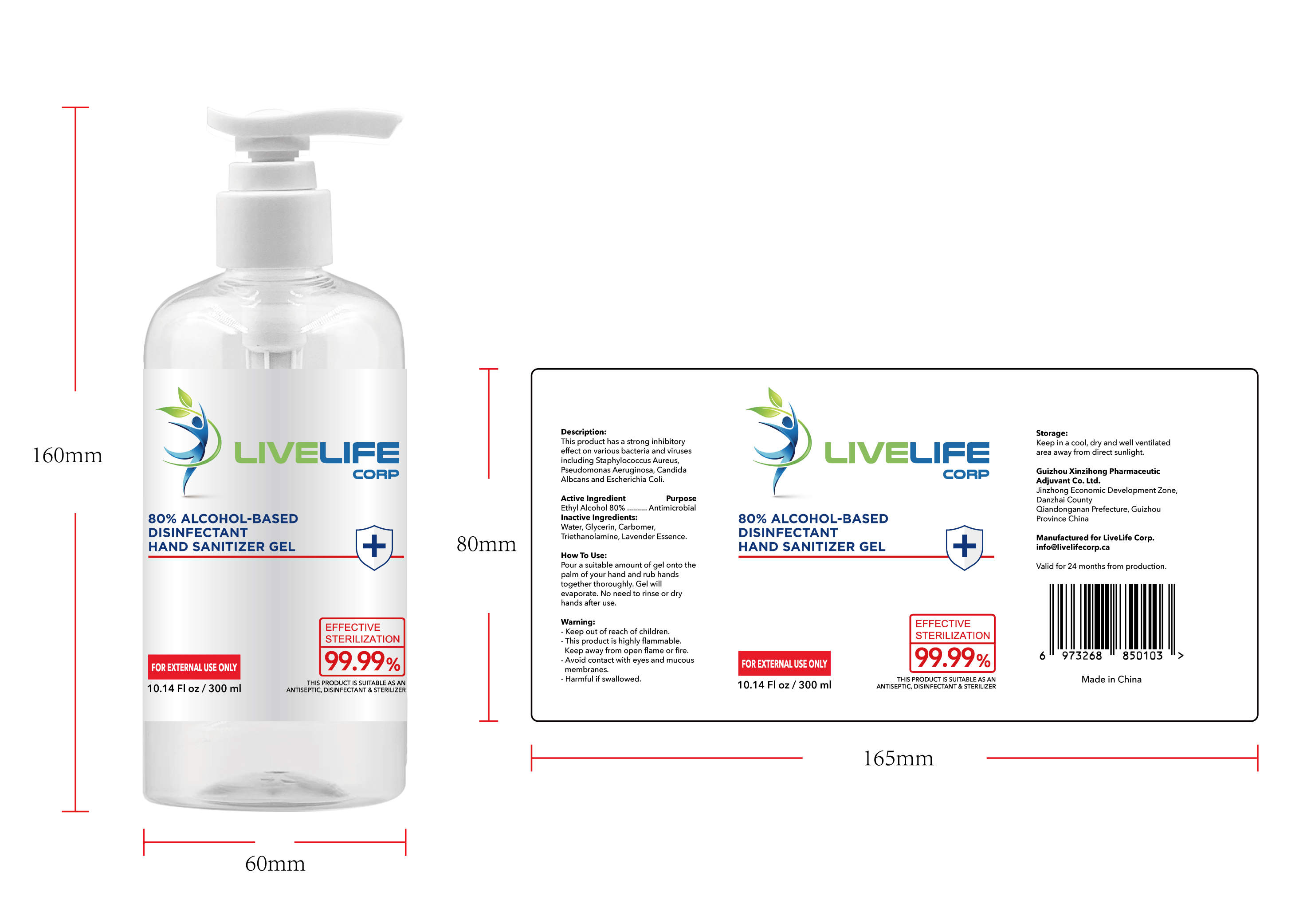
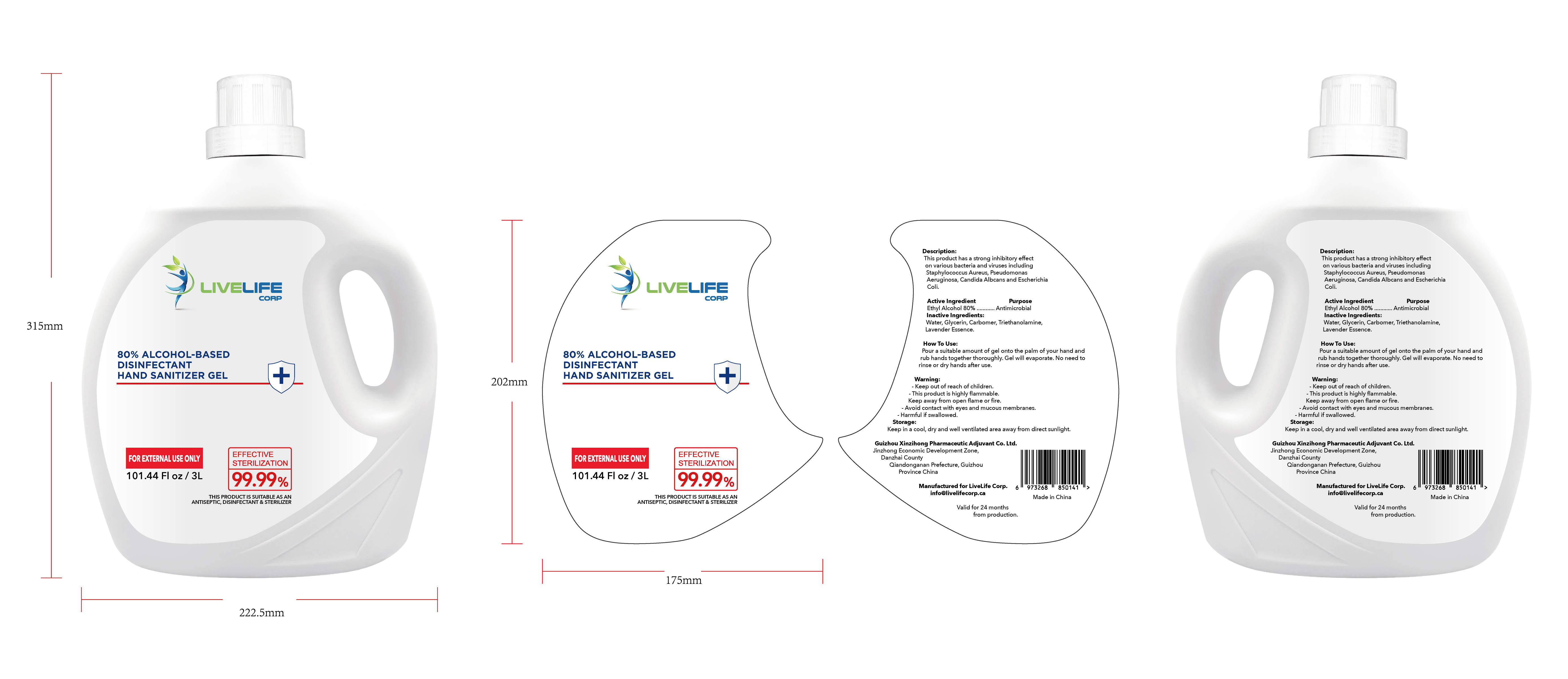
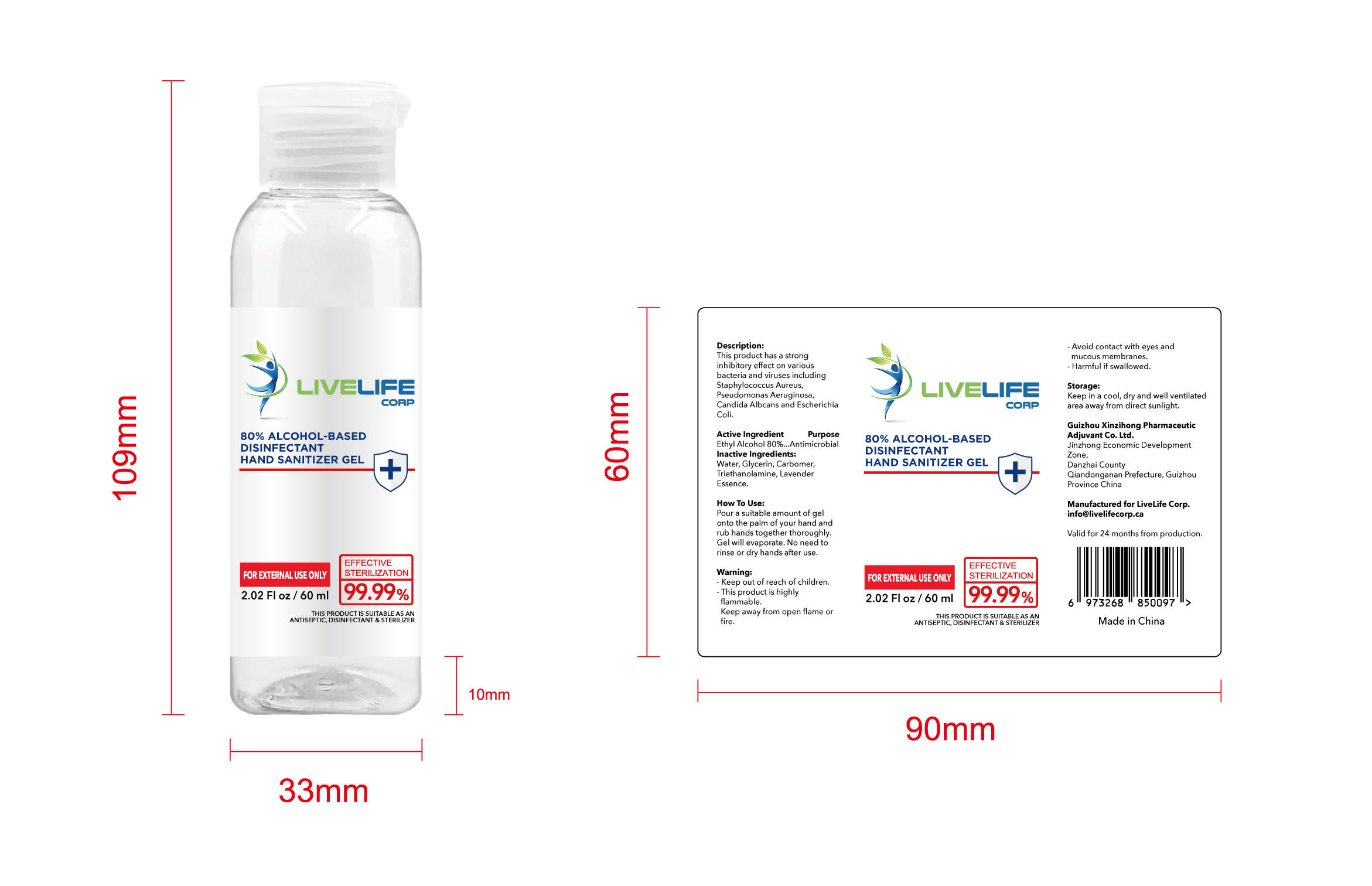
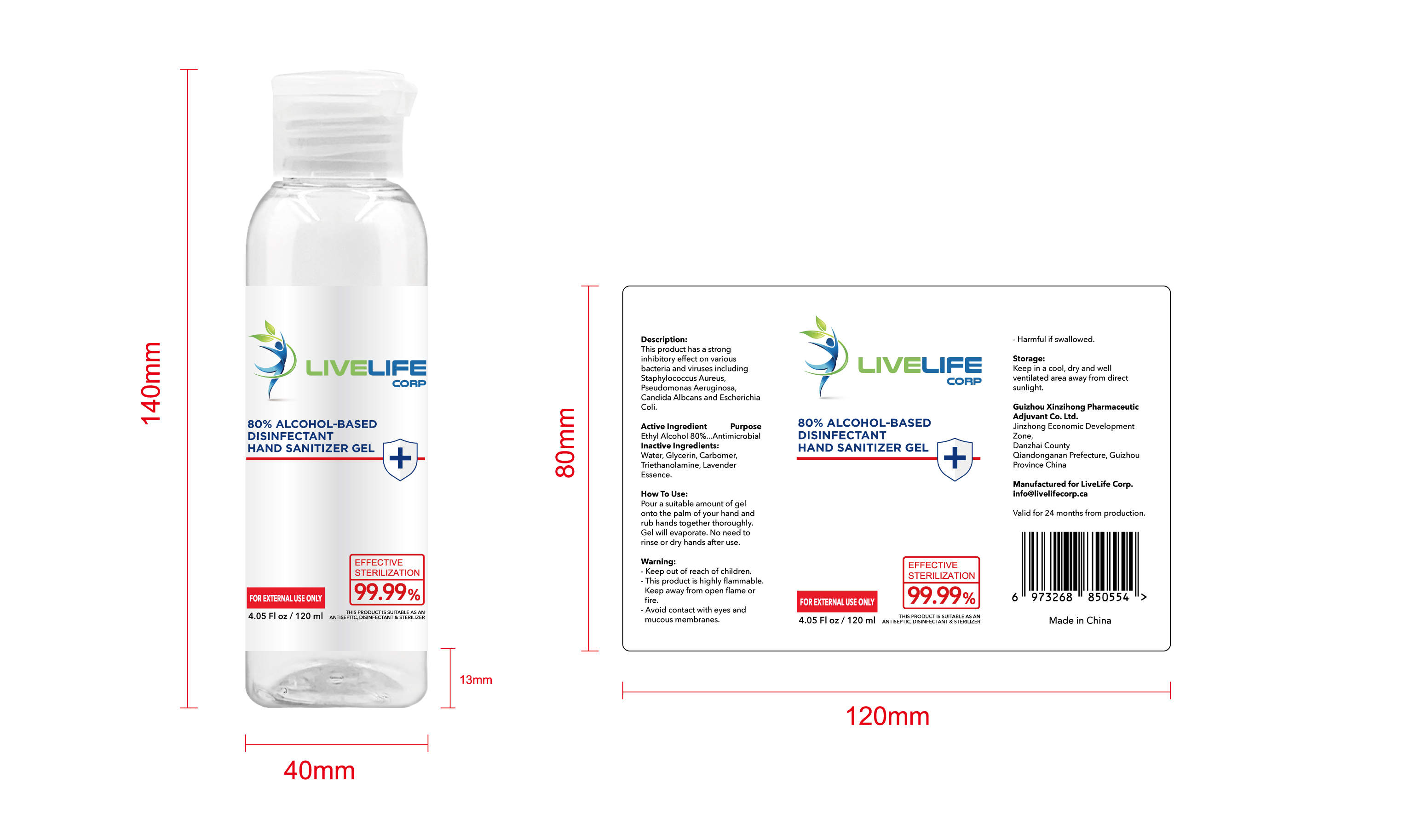
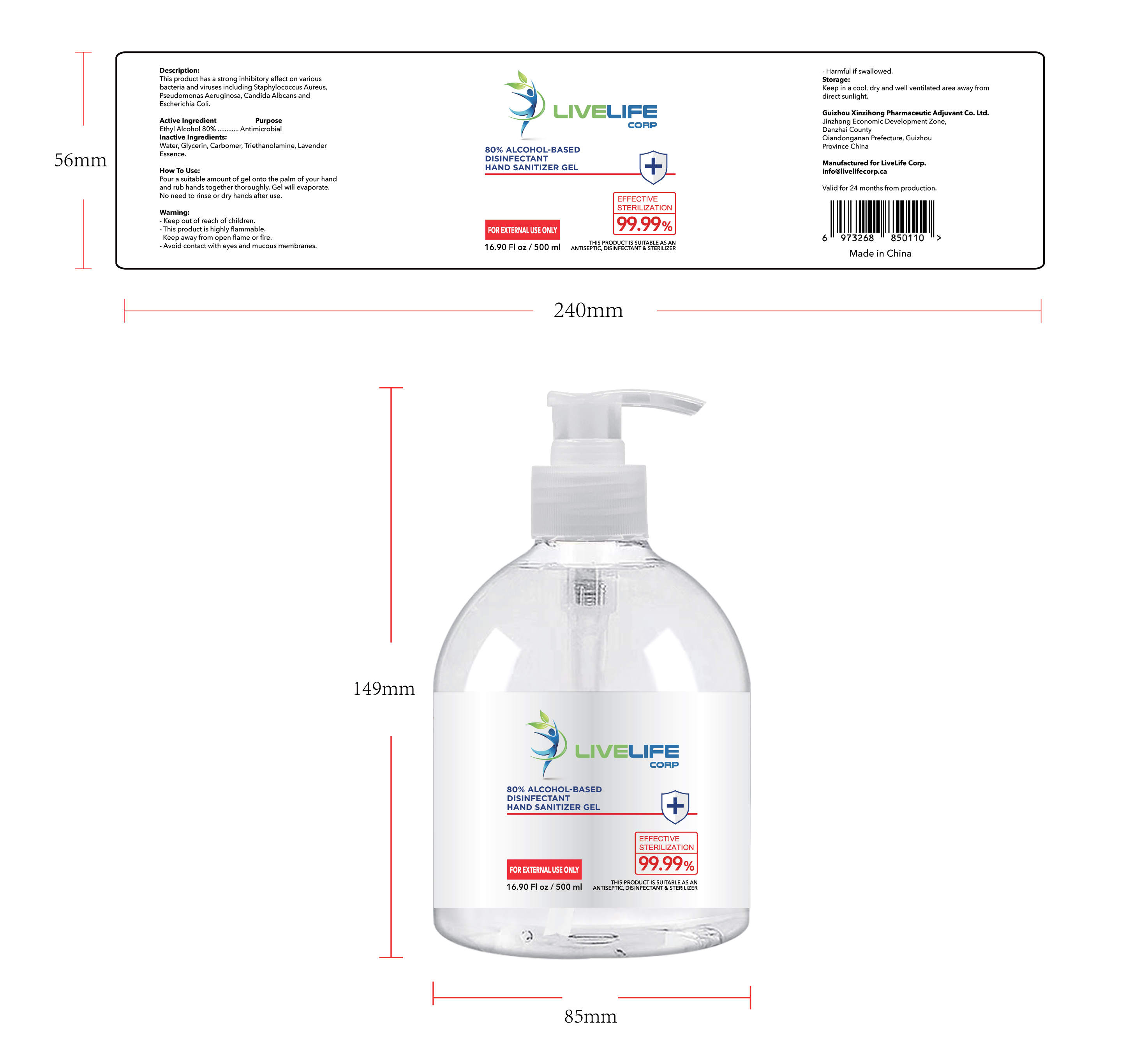
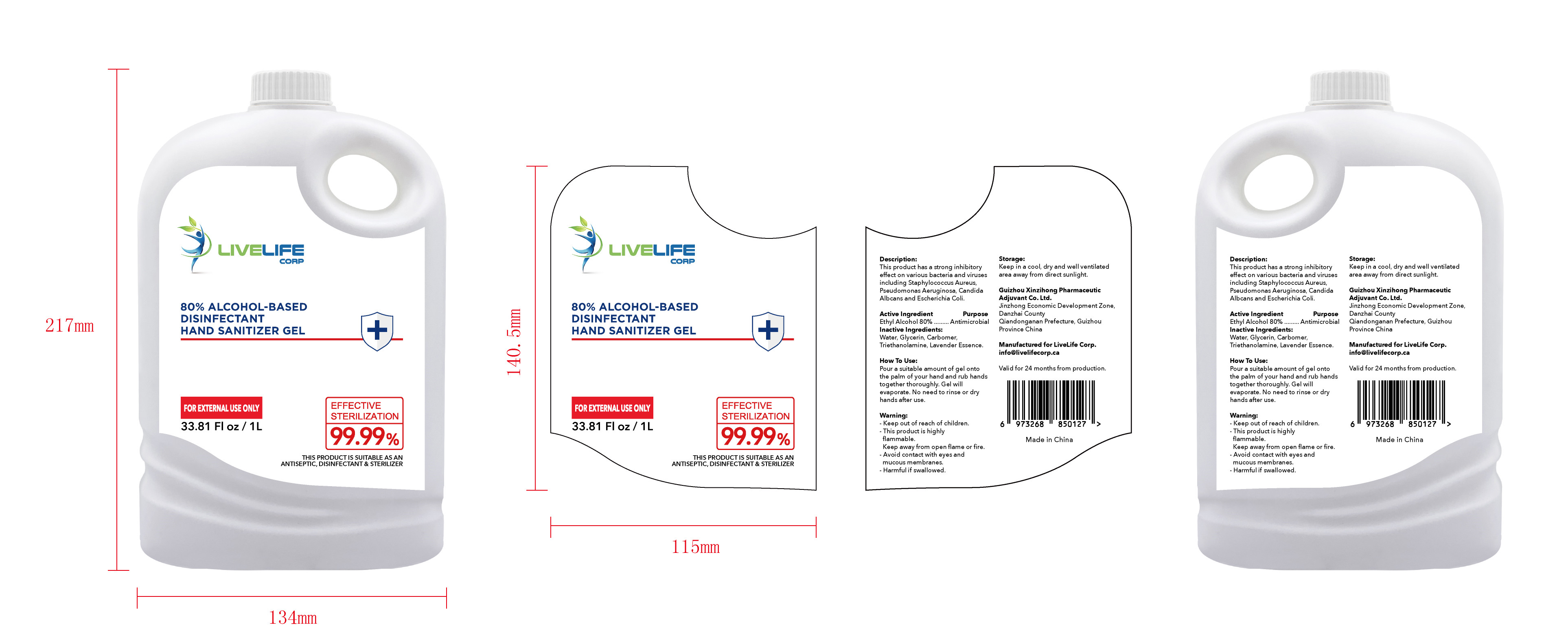
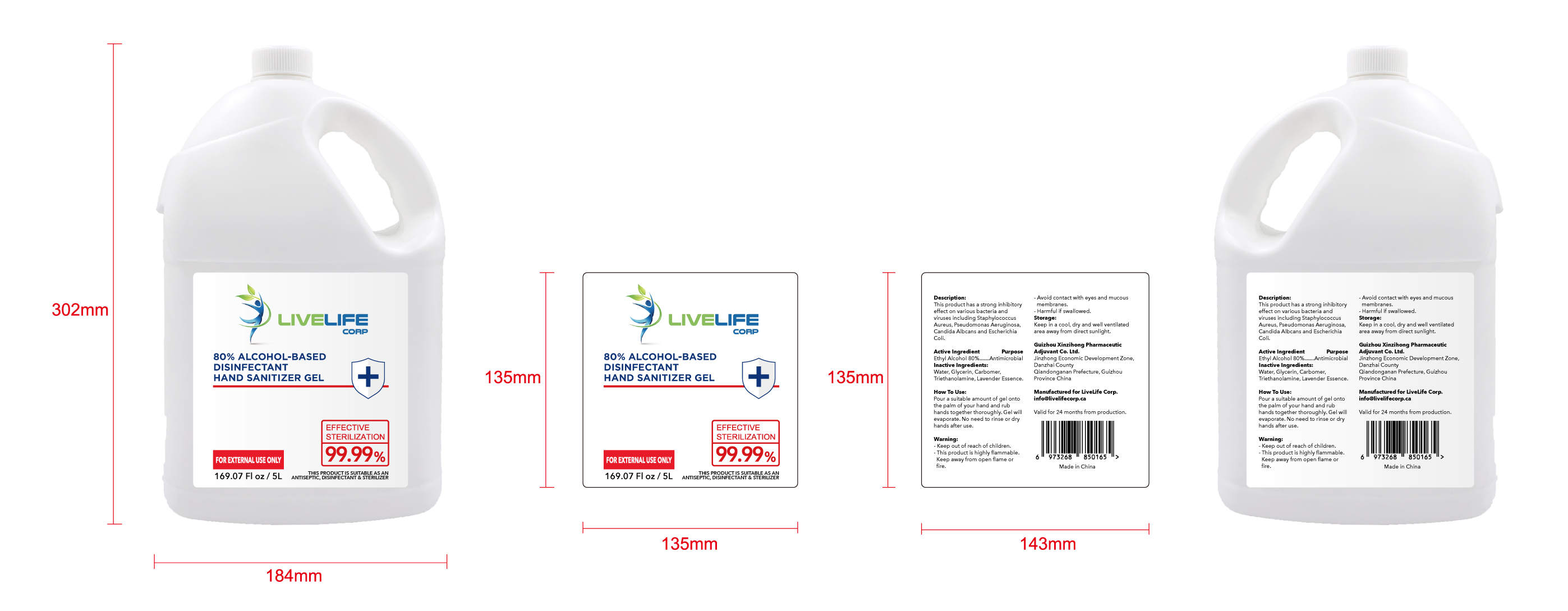
ALCOHOL-BASED DISINFECTANT HAND SANITIZER- alcohol-based disinfectant hand sanitizer gel
Foshan Lang-Yeon Trade Limited Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient(s)
Ethyl Alcohol 75% v/v. Purpose: Antimicrobial
Purpose
Purpose: Antimicrobial , Hand Sanitizer
Use
Pour a suitable amount of gel onto the palm of your hand and rub hands together thoroughly. Gel will evaporate. No need to rinse or dry hands after use
Warnings
Keep out of reach of children This product is highly flammable Keep away from open flame or fire Avoid contact with eyes and mucous membranes Harmful if swallowed
Directions
This product has a strong inhibitoryffect on various bacteria and virusesincluding Staphylococcus Aureus,
Pseudomonas Aeruginosa, CandidaAlbcans and Escherichia Coli
Other information
Keep in a cool, dry and well ventilated area away from direct sunlight
Inactive ingredients
Water, Glycerin, Carbomer,Triethanolamine, Lavender Essence