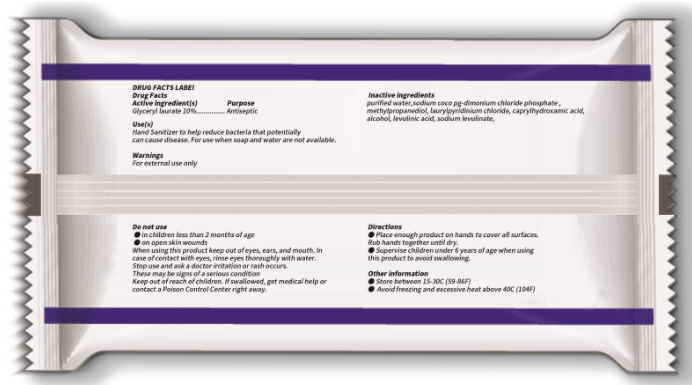
Use
Hand Sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
When using this product
keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
● Place enough product on hands to cover all surfaces. Rub hands together until dry.
● Supervise children under 6 years of age when using this product to avoid swallowing.
Other information
● Store between 15-30C (59-86F)
● Avoid freezing and excessive heat above 40C (104F)