FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Adapalene and benzoyl peroxide gel pad 0.1% / 2.5% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
2 DOSAGE AND ADMINISTRATION
For topical use only; adapalene and benzoyl peroxide gel pad is not for oral, ophthalmic, or intravaginal use. Apply adapalene and benzoyl peroxide gel pad to affected areas of the face and/or trunk once daily after washing. Use pad for area of the face (e.g., forehead, chin, each cheek). Avoid the eyes, lips and mucous membranes.
3 DOSAGE FORMS AND STRENGTHS
Adapalene and benzoyl peroxide gel pad contains 1 mg (0.1%) adapalene, USP and 25 mg (2.5%) benzoyl peroxide, USP.
5 WARNINGS AND PRECAUTIONS
5.1 Ultraviolet Light and Environmental Exposure
Exposure to sunlight, including sunlamps, should be minimized during the use of adapalene and benzoyl peroxide gel pad. Patients with high levels of sun exposure and those with inherent sensitivity to sun should exercise particular caution. Use of sunscreen products and protective apparel, (e.g., hat) are recommended when exposure cannot be avoided. Weather extremes, such as wind or cold, may be
irritating to patients under treatment with adapalene and benzoyl peroxide gel pad.
5.2 Local Cutaneous Reactions
Erythema, scaling, dryness, and stinging/burning may be experienced with use of adapalene and benzoyl peroxide gel pad. These are most likely to occur during the first four weeks of treatment, are mostly mild to moderate in intensity, and usually lessen with continued use of the medication. Irritant and allergic contact dermatitis may occur. Depending upon the severity of these adverse reactions, patients should be instructed to use a moisturizer, reduce the frequency of the application of adapalene and benzoyl peroxide gel pad, or discontinue use. The product should not be applied to cuts, abrasions, eczematous or sunburned skin. As with other retinoids, use of “waxing” as a depilatory method should be avoided on skin treated with adapalene and benzoyl peroxide gel pad. Avoid concomitant use of other potentially irritating topical products (medicated or abrasive soaps and cleansers, soaps and cosmetics that have strong skin-drying effect and products with high concentrations of alcohol, astringents, spices, or limes).
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
During clinical trials, 1401 subjects were exposed to adapalene and benzoyl peroxide. A total of 1036 subjects with acne vulgaris, 12 years and older, were treated once daily for 12 weeks to 12 months. Related adverse events reported within 12 weeks of treatment and in at least 1% of subjects treated with adapalene and benzoyl peroxide and those reported in subjects treated with the vehicle are presented in
Table 1:
Table 1. Drug Related Adverse Events Reported in Clinical Trials by At Least 1% of Patients Treated For 12 Weeks
|
System Organ Class/ Preferred Term |
Adapalene and Benzoyl Peroxide N=564 |
Vehicle N=489 |
| Subjects with AE (s) | 14% | 4% |
| Dry Skin | 7% | 2% |
| Contact dermatitis | 3% | <1% |
| Application site burning | 2% | <1% |
| Application site irritation | 1% | <1% |
| Skin irritation | 1% | 0% |
Local tolerability evaluations, presented in Table 2, were conducted at each study visit in clinical trials by assessment of erythema, scaling, dryness, burning, and stinging.
Table 2. Incidence of Local Cutaneous Irritation in Controlled Clinical Trials (N=553) Treatment Emergent Signs and Symptoms
|
Maximum Severity During Treatment |
End of Treatment Severity (12 Weeks) |
|||||
|
Mild |
Moderate |
Severe |
Mild |
Moderate |
Severe |
|
|
Erythema |
27% |
13% |
1% |
8% |
2% |
1% |
|
Scaling |
35% |
11% |
1% |
9% |
1% |
<1% |
|
Dryness |
41% |
13% |
1% |
10% |
2% |
<1% |
|
Stinging/burning |
41% |
15% |
3% |
7% |
2% |
1% |
Analysis over the 12 week period showed that local tolerability scores for erythema, scaling, dryness, and stinging/burning peaked at Week 1 of therapy and decreased thereafter.
During a pediatric clinical trial, 285 children with acne vulgaris, 9 to 11 years of age were treated with adapalene and benzoyl peroxide or with the vehicle once daily for 12 weeks. Overall, the safety profile of adapalene and benzoyl peroxide in these subjects is comparable to the safety profile observed in older subjects 12 years of age and above, both in the nature and frequency of the observed events.
Analysis of local tolerability evaluations shows similar incidence of treatment emergent signs and symptoms as in subjects 12 years of age and above, with local tolerability signs and symptoms peaking during the first week and decreasing over time.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of adapalene and benzoyl peroxide: eyelid edema, sunburn, blister, pain of skin, pruritus, swelling face, conjunctivitis, skin discoloration, rash, eczema, throat tightness and allergic contact dermatitis. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7 DRUG INTERACTIONS
Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating, or abrasive agents. No formal drugdrug interaction studies were conducted with adapalene and benzoyl peroxide.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C
There are no well-controlled trials in pregnant women treated with adapalene and benzoyl peroxide. Animal reproduction studies have not been conducted with the combination or benzoyl peroxide.
Furthermore, such studies are not always predictive of human response; therefore, adapalene and benzoyl peroxide should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
No teratogenic effects were observed in rats treated with oral doses of 0.15 to 5 mg adapalene/kg/day, up to 25 times (mg/m
2/day) the maximum recommended human dose (MRHD) of 2 grams of adapalene and benzoyl peroxide. However, teratogenic changes were observed in rats and rabbits when treated with oral doses of greater than or equal to 25 mg adapalene/kg/day representing 123 and 246 times
MRHD, respectively. Findings included cleft palate, microphthalmia, encephalocele and skeletal abnormalities in rats; and umbilical hernia, exophthalmos and kidney and skeletal abnormalities in rabbits.
Dermal teratology studies conducted in rats and rabbits at doses of 0.6 to 6 mg adapalene/kg/day [25 to 59 times (mg/m 2) the MRHD] exhibited no fetotoxicity and only minimal increases in supernumerary ribs in both species and delayed ossification in rabbits.
8.3 Nursing Mothers
It is not known whether adapalene or benzoyl peroxide is excreted in human milk following use of adapalene and benzoyl peroxide. Because many drugs are excreted in human milk, caution should be exercised when adapalene and benzoyl peroxide is administered to a nursing woman.
11 DESCRIPTION
Adapalene and benzoyl peroxide, 0.1% / 2.5% is a 1.2g gel pad for topical use containing adapalene USP, 0.1% and benzoyl peroxide USP, 2.5%.
Adapalene USP, a synthetic retinoid, is a naphthoic acid derivative with retinoid-like properties. The chemical name for adapalene, USP is (6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid). It has the following structural formula:
Adapalene, USP:
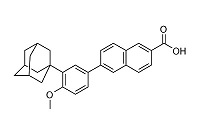
Molecular formula: C 28H 28O 3 Molecular weight: 412.5
Benzoyl Peroxide, USP is a highly lipophilic oxidizing agent that localizes in both bacterial and keratinocyte cell membranes.
The chemical name for benzoyl peroxide, USP is dibenzoyl peroxide. It has the following structural formula:
Benzoyl Peroxide, USP:
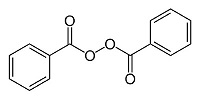
Molecular formula: C 14H 10O 4 Molecular weight: 242.23
Inactive ingredients: carbomer 980, docusate sodium, edetate disodium, glycerin, poloxamer 182, polysorbate 80, propylene glycol, purified water, sorbitan monooleate and xanthan gum.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Adapalene
Adapalene binds to specific retinoic acid nuclear receptors but does not bind to cytosolic receptor protein. Biochemical and pharmacological profile studies have demonstrated that adapalene is a modulator of cellular differentiation, keratinization and inflammatory processes. However, the significance of these findings with regard to the mechanism of action of adapalene for the treatment of acne is unknown.
Benzoyl peroxide
Benzoyl peroxide is an oxidizing agent with bactericidal and keratolytic effects.
12.3 Pharmacokinetics
A pharmacokinetic study was conducted in 10 adult subjects with acne vulgaris who were treated once daily for 30 days with 2 grams/day of adapalene and benzoyl peroxide applied to 1000 cm 2 of acne involved skin, (face, chest, and upper back). Two subjects (20%) had quantifiable adapalene plasma concentrations above the limit of quantification (LOQ=0.1ng/mL). The highest adapalene C max and AUC 0-24h was 0.21 ng/mL and 1.99 ng•h/mL, respectively. Excretion of adapalene appears to be primarily by the biliary route. Pharmacokinetics of adapalene and benzoyl peroxide in pediatric subjects have not been evaluated.
Benzoyl peroxide is absorbed by the skin where it is converted to benzoic acid and eliminated in the urine.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity, photocarcinogenicity, genotoxicity, or fertility studies were conducted with adapalene and benzoyl peroxide. Carcinogenicity studies with adapalene have been conducted in mice at topical doses of 0.4, 1.3, and 4.0 mg/kg/day (1.2, 3.9, and 12 mg/m 2/day), and in rats at oral doses of 0.15, 0.5, and 1.5 mg/kg/day (0.9, 3.0, and 9.0 mg/m 2/day). In terms of body surface area, the highest dose levels are 9.8 (mice) and 7.4 times (rats) the MRHD of 2 grams of adapalene and benzoyl peroxide. In the rat study, an increased incidence of benign and malignant pheochromocytomas in the adrenal medulla of male rats was observed.
No significant increase in tumor formation was observed in rodents topically treated with 15 to 25% benzoyl peroxide carbopol (6 to 10 times the concentration of benzoyl peroxide in adapalene and benzoyl peroxide) for two years. Rats received maximum daily applications of 138 (males) and 205 (females) mg benzoyl peroxide/kg. In terms of body surface area, these levels are 27 to 40 times the MRHD. Similar results were obtained in mice topically treated with 25% benzoyl peroxide carbopol for 56 weeks followed by intermittent treatment with 15% benzoyl peroxide carbopol for rest of the 2 year study period, and in mice topically treated with 5% benzoyl peroxide carbopol for two years.
The role of benzoyl peroxide as a tumor promoter has been well established in several animal species. However, the significance of this finding in humans is unknown.
In a photocarcinogenicity study conducted with 5% benzoyl peroxide carbopol, no increase in UV-induced tumor formation was observed in hairless mice topically treated for 40 weeks.
No photocarcinogenicity studies were conducted with adapalene. However, animal studies have shown an increased tumorigenic risk with the use of pharmacologically similar drugs (e.g., retinoids) when exposed to UV irradiation in the laboratory or sunlight. Although the significance of these findings to humans is not clear, patients should be advised to avoid or minimize exposure to either sunlight or artificial irradiation sources.
Adapalene did not exhibit mutagenic or genotoxic effects in vitro (Ames test, Chinese hamster ovary cell assay, mouse lymphoma TK assay) or in vivo (mouse micronucleus test).
Bacterial mutagenicity assays (Ames test) with benzoyl peroxide has provided mixed results, mutagenic potential was observed in a few but not in a majority of investigations. Benzoyl peroxide has been shown to produce single-strand DNA breaks in human bronchial epithelial and mouse epidermal cells, it has caused DNA-protein cross-links in the human cells, and has also induced a dose-dependent increase in sister chromatid exchanges in Chinese hamster ovary cells.
In rat oral studies, 20 mg adapalene/kg/day (120 mg/m 2/day; 98 times the MRHD based on mg/m 2/day comparison) did not affect the reproductive performance and fertility of F 0 males and females, or growth, development and reproductive function of F 1 offspring.
No fertility studies were conducted with benzoyl peroxide.
14 CLINICAL STUDIES
The safety and efficacy of adapalene and benzoyl peroxide applied once daily for the treatment of acne vulgaris were assessed in two 12 week, multicenter, controlled clinical studies of similar design, comparing adapalene and benzoyl peroxide to the vehicle in acne subjects. Treatment response was defined as the percent of subjects who had a two grade improvement and rated ‘Clear’ and ‘Almost Clear’ at Week 12 based on the Investigator’s Global Assessment (IGA) and mean absolute change from baseline at Week 12 in both inflammatory and non-inflammatory lesion counts. An IGA score of ‘Clear’ corresponded to residual hyperpigmentation and erythema may be present. An IGA score of ‘Almost Clear’ corresponded to a few scattered comedones and a few small papules.
In Study 1, 517 subjects were randomized to adapalene and benzoyl peroxide, adapalene 0.1% in vehicle, benzoyl peroxide 2.5% in vehicle. The median age of these 517 subjects was 15 years old and 60% were males.
At baseline, subjects had between 20 to 50 inflammatory lesions and 30 to 100 non-inflammatory lesions. The majority of subjects had a baseline IGA score of ‘Moderate’ which corresponded to more than half of the face is involved, many comedones, papules and pustules. The efficacy results at week 12 are presented in Table 3.
In Study 2, 1668 subjects were randomized to adapalene and benzoyl peroxide, adapalene 0.1% in vehicle, benzoyl peroxide 2.5% in vehicle. The median age of subjects was 16 years old and 49% were males. At baseline, subjects had between 20 to 50 inflammatory lesions and 30 to 100 non-inflammatory lesions as well as an Investigator Global
Assessment score of ‘Moderate’. The efficacy results at week 12 are presented in Table 3.
In study 3, 285 pediatric subjects 9 to 11 years of age were randomized to adapalene and benzoyl peroxide or vehicle. The median age of subjects was 11 years and 24% were males. At baseline, subjects had a minimum of 20 but not more than 100 total lesions (inflammatory and/or non-inflammatory) with an Investigator Global Assessment score of ‘Moderate’. The efficacy results at week 12 are presented in Table 3.
Table 3: Clinical Efficacy of Adapalene and Benzoyl Peroxide Gel 0.1% / 2.5% at Week 12
|
Study 1 |
||||
|
Adapalene and Benzoyl Peroxide Gel (N=149) |
Adapalene 0.1% in Vehicle gel (N=148) |
Benzoyl Peroxide 2.5% in Vehicle gel (N=149) |
Vehicle Gel (N=71) |
|
|
IGA: Two Grade Improvement and Clear or Almost Clear |
32 (21.5%) |
18 (12.2%) |
18 (12.1%) |
4 (5.6%) |
|
Inflammatory Lesions: Mean Absolute (Percent) Change |
16.0 (52.4%) |
11.4 (39.9%) |
10.5 (35.8%) |
9.5 (31.8%) |
|
Non-inflammatory Lesions: Mean Absolute (Percent) Change |
23.4 (45.9%) |
15.2 (29.6%) |
13.7 (32.2%) |
13.2 (27.8%) |
|
Study 2 |
||||
|
Adapalene and Benzoyl Peroxide Gel (N=415) |
Adapalene 0.1% in Vehicle gel (N=420) |
Benzoyl Peroxide 2.5% in Vehicle gel (N=415) |
Vehicle Gel (N=418) |
|
|
IGA: Two Grade Improvement and Clear or Almost Clear |
125 (30.1%) |
83 (19.8%) |
92 (22.2%) |
47 (11.3%) |
|
Inflammatory Lesions: Mean Absolute (Percent) Change |
15.4 (53.4%) |
12.3 (41.7%) |
13.7 (47.6%) |
8.7 (30.2%) |
|
Non-inflammatory Lesions: Mean Absolute (Percent) Change |
24.6 (48.1%) |
21.0 (40.8%) |
19.2 (37.2%) |
11.3 (23.2%) |
In both Studies 1 and 2 the treatment effect was smaller in subjects with a small number of baseline lesions than in subjects with a large number of baseline lesions.
|
Study 3 |
||
|
Adapalene and Benzoyl Peroxide N=142 |
Vehicle N=143 |
|
|
IGA: Two Grade Improvement and Clear or Almost Clear |
67 (47.2%) |
22 (15.4%) |
|
Inflammatory Lesions: Mean Absolute (Percent) Change |
7.4 (36.0%) |
0.7 (-13.2%) * |
|
Non-inflammatory Lesions: Mean Absolute (Percent) Change |
20.2 (54.7%) |
2.9 (2.3%) |
*That is, a mean percent increase of 13.2%
16 HOW SUPPLIED/STORAGE AND HANDLING
Adapalene and benzoyl peroxide gel pad 0.1% / 2.5% is supplied in the following size:
14 count use-of-use 1.2g gel pads NDC 82429-012-01
STORAGE AND HANDLING
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Protect from light and keep away from heat. Keep tube tightly closed.
Keep out of reach of children.
17 PATIENT COUNSELING INFORMATION
Information for Patients
- Advise patients to cleanse the area to be treated with a mild or soapless cleanser; pat dry. Apply adapalene and benzoyl peroxide as a pad, avoiding the eyes, lips and mucous membranes.
- Advise patients not to use more than the recommended amount and not to apply more than once daily as this will not produce faster results but may increase irritation.
- Adapalene and benzoyl peroxide may cause irritation such as erythema, scaling, dryness, stinging or burning.
- Advise patients to minimize exposure to sunlight, including sunlamps. Recommend the use of sunscreen products and protective apparel, (e.g., hat) when exposure cannot be avoided. Adapalene and benzoyl peroxide pad may bleach hair and colored fabric.
PATIENT INFORMATION
Adapalene (a dap' a leen) and Benzoyl Peroxide (ben' zoe il per ox' ide) Gel Pad 0.1%/2.5%
Rx Only
Important: For use on the skin only (topical). Do not use adapalene and benzoyl peroxide in or on your mouth, eyes, or vagina.
Read this Patient Information leaflet about adapalene and benzoyl peroxide before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your treatment or your medical condition. If you have any questions about adapalene and benzoyl peroxide, talk with your doctor or pharmacist.
What is adapalene and benzoyl peroxide?
Adapalene and benzoyl peroxide is a prescription medicine for skin use only (topical) used to treat acne vulgaris in people 9 years of age and older.
Acne vulgaris is a condition in which the skin has blackheads, whiteheads and pimples.
It is not known if adapalene and benzoyl peroxide is safe and effective in children younger than 9 years old.
What should I tell my doctor before using adapalene and benzoyl peroxide gel pad?
Before you use adapalene and benzoyl peroxide gel pad, tell your doctor if you:
- have other skin problems, including cuts or sunburns
- have any other medical conditions
- are pregnant or planning to become pregnant. It is not know if adapalene and benzoyl peroxide gel pad can harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if adapalene and benzoyl peroxide passes into your breast milk and if it can harm your baby. Talk to your doctor about the best way to feed your baby if you use adapalene and benzoyl peroxide gel pad.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements.
Especially tell your doctor if you use any other medicine for acne. Using adapalene and benzoyl peroxide gel pad with topical medicines that contain sulfur, resorcinol or salicylic acid may cause skin irritation.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use adapalene and benzoyl peroxide gel pad?
- Use adapalene and benzoyl peroxide gel pad exactly as your doctor tells you to use it. Adapalene and benzoyl peroxide gel pad is for skin use only. Do not use adapalene and benzoyl peroxide gel pad in or on your mouth, eyes, or vagina.
- Apply adapalene and benzoyl peroxide gel pad 1 time a day.
- Do not use more adapalene and benzoyl peroxide gel pad than you need to cover the treatment area. Using too much adapalene and benzoyl peroxide gel pad or using it more than 1 time a day may increase your chance of skin irritation.
Applying adapalene and benzoyl peroxide gel pad:
- Wash the area where the pad will be applied with a mild cleanser and pat dry.
- Adapalene and benzoyl peroxide gel pad comes in a 14 count unit of use 1.2g gel pads. If you have been prescribed the: Pad: Remove the pad from the foil before using. Adapalene pad should be applied once a day.
What should I avoid while using adapalene and benzoyl peroxide gel pad?
- You should avoid spending time in sunlight or artificial sunlight, such as tanning beds or sunlamps. Adapalene and benzoyl peroxide pad can make your skin sensitive to sun and the light from tanning beds and sunlamps. You should wear sunscreen and wear a hat, and clothes that cover the areas treated with adapalene and benzoyl peroxide pad if you have to be in sunlight.
- You should avoid weather extremes such as wind and cold as this may cause irritation to your skin.
- You should avoid applying adapalene and benzoyl peroxide pad to cuts, abrasions and sunburned skin.
- You should avoid skin products that may dry or irritate your skin such as harsh soaps, astringents, cosmetics that have strong skin drying effects and products containing high levels of alcohol. You should avoid the use of “waxing” as a hair removal method on skin treated with adapalene and benzoyl peroxide gel pad.
- Adapalene and benzoyl peroxide gel pad may bleach your clothes or hair. Allow adapalene and benzoyl peroxide pad to dry completely before dressing to prevent bleaching of your clothes.
What are the possible side effects of adapalene and benzoyl peroxide gel pad?
Adapalene and benzoyl peroxide gel pad may cause serious side effects including:
- Local skin reactions. Local skin reactions are most likely to happen during the first 4 weeks of treatment and usually lessen with continued use of adapalene and benzoyl peroxide gel pad. Signs and symptoms of local skin reaction include:
- Redness
- Dryness
- Swelling
- Scaling
- Stinging or burning
Tell your doctor right away if these side effects continue for longer than 4 weeks or get worse, you may have to stop using adapalene and benzoyl peroxide gel pad.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of adapalene and benzoyl peroxide gel pad. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Gabar Health Sciences Corp at 1-470-737-9424
How should I store adapalene and benzoyl peroxide gel pad?
- Store adapalene and benzoyl peroxide gel pad at room temperature, 68° to 77° F (20° to 25° C)
- Keep adapalene and benzoyl peroxide gel pad inside container and out of light and away from heat.
Keep adapalene and benzoyl peroxide gel pad and all medicines out of the reach of children.
General information about adapalene and benzoyl peroxide gel pad
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use adapalene and benzoyl peroxide gel pad for a condition for which it was not prescribed. Do not give adapalene and benzoyl peroxide gel pad to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about adapalene and benzoyl peroxide gel pad. If you would like more information, talk with your doctor. You can also ask your doctor or pharmacist for information about adapalene and benzoyl peroxide gel pad that is written for health professionals.
What are the ingredients in adapalene and benzoyl peroxide gel pad?
Active ingredient: adapalene, USP and benzoyl peroxide, USP
Inactive ingredients: carbomer 980, docusate sodium, edetate disodium, glycerin, poloxamer 182, polysorbate 80, propylene glycol, purified water, sorbitan monooleate and xanthan gum.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured and distributed by:
Gabar Health Sciences Corp, Atlanta, GA 30354 USA
Revised - August 2022 20203912
