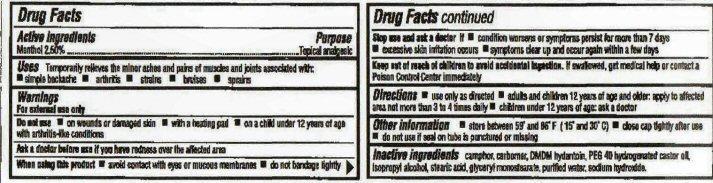
Keep out of reach of children
Keep out of reach of children to avoid accidental ingestion. If swallowed, get medical help or contact a Poison Control Center immediately.
Uses
Temporarily relieves the minor aches and pains of muscles and joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only.
Do not use on
- on wounds or damaged skin
- with a heating pad
- on a child under 12 years of age with arthritis-like conditions
Ask a doctor before use if you have redness over the affected area.
When using this product
- avoid contact with eyes or mucus membranes
- do not bandage tightly
Stop use and ask a doctor if
- condition worsens or symptoms persist for more than 7 days
- excessive skin irritation occurs
- symptoms clear up and occur again within a few days
Directions
- use only as directed
- adults and children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: ask a doctor
Other information
- store between 59° and 86° F (15° and 30° C)
- close cap tightly after use
- do not use if seal on tube is punctured or missing
Inactive ingredients
camphor, carbomer, DMDM hydantoin,PEG-40 hydrogenated castor oil, isopropyl alcohol, stearic acid, glyceryl monostearate, purified water, sodium hydroxide.
Product Label
DRUGSTORE-Rx
SMART WELLNESS
Ultra Strength
Muscle Rub
Pain Relieving Cream Analgesic
Relieves minor
- Arthritis
- Backache
- Muscle pain
- Joint pain
Fast penetrating
Long lasting
NET WT. 1.5 OZ. (42 g)
DRUGSTORE-Rx
SMART WELLNESS delivers outstanding quality in healthcare products, at truly amazing prices. We employ the highest standard when searching the world to bring the best value to your doorstep. Use our products confidently, knowing that attention and care has gone into every item. That's smart wellness!
Distributed by: PERSONAL CARE PRODUCTS, LLC
Troy, Michigan 48084 U.S.A.
Code No. MH/DRUGS/KD-313 Made in India