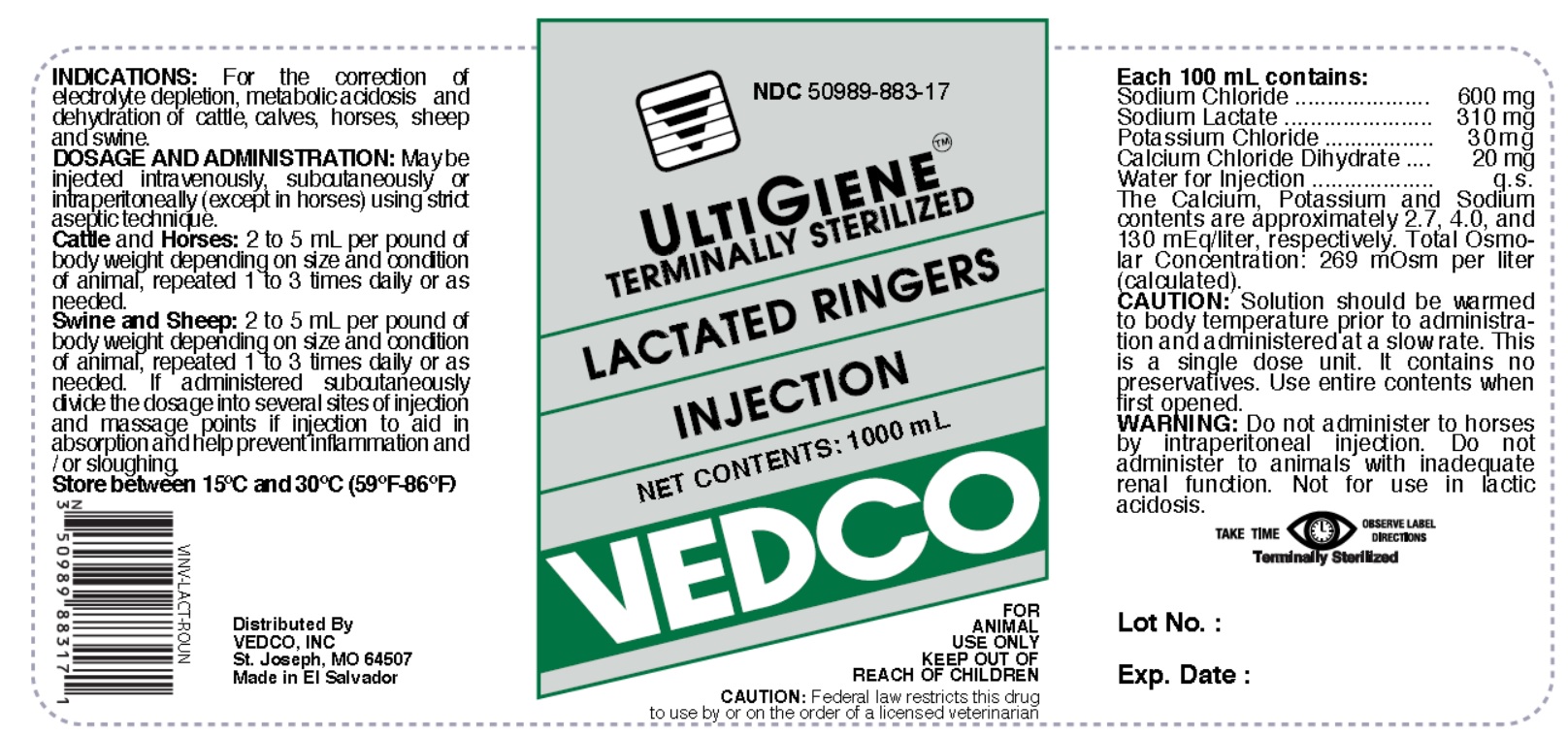
LACTATED RINGERS- lactated ringers injection, solution
Vedco
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA.
LACTATED RINGERS INJECTION
ACTIVE INGREDIENTS
Each 100 mL contains:
Sodium Chloride ....................... 600 mg
Sodium Lactate ........................ 310 mg
Potassium Chloride .................... 30 mg
Calcium Chloride Dihydrate ......... 20 mg
Water for Injection ........................ q.s.
The Calcium, Potassium and Sodium contents are approximately 2.7, 4.0, and 130 mEq/liter, respectively. Total Osmolar Concentration: 269 mOsm per liter (calculated).
INDICATIONS
For the correction of electrolyte depletion, metabolic acidosis and dehydration of cattle, calves, horses, sheep and swine.
DOSAGE AND ADMINISTRATION:
May be injected intravenously, subcutaneously or intraperitoneally (except in horses) using strict aseptic technique.
Cattle and Horses: 2 to 5 mL per pound of body weight depending on size and condition of animal, repeated 1 to 3 times daily or as needed.
Swine and Sheep: 2 to 5 mL per pound of body weight depending on size and condition of animal, repeated 1 to 3 times daily or as needed.
If administered subcutaneously divide the dosage into several sites of injection and massage points of injection to aid in absorption and help prevent inflammation and / or sloughing.
CAUTION:
Solution should be warmed to body temperature prior to administration and administered at a slow rate. This is a single dose unit. It contains no preservatives. Use entire contents when first opened.
WARNING:
Do not administer to horses by intraperitoneal injection. Do not administer to animals with inadequate renal function. Not for use in lactic acidosis.