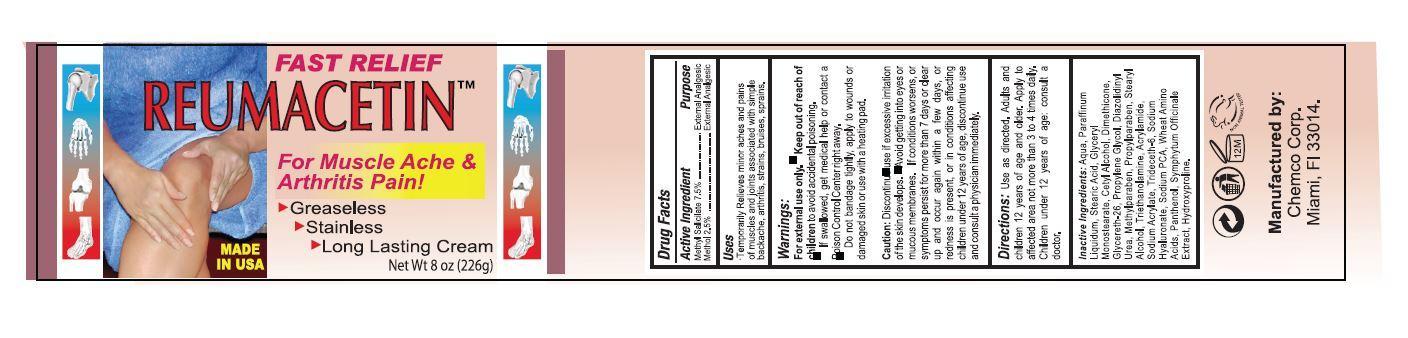
DRUG FACTS
Active Ingredient: Purpose:
Methyl Salicylate 7.5%......................External Analgesic
Menthol 2.5%.................................External Analgesic
Uses
Temporarily relieves minor aches and pains of muscles and joints associated with simple backache, arthritis, strains, bruises, sprains.
Warnings:
- For external use only.
- If swallowed, get medical help or contact a Poison Control Center right away.
- Do not bandage tightly, apply to wounds or damaged skin or use with a heating pad.
Caution: Discontinue use if excessive irritation of the skin develops. Avoid getting into eyes or mucous membranes. If condition worsens, or symptoms persist for more than 7 days or clear up and occur again within a few days, or redness is present, or in conditions affecting children under 12 years of age, discontinue use and consult a physician immediately.
Directions
Use as directed. Adults and children 12 years of age and older. Apply to affected area not more than 3 to 4 times daily. Children under 12 years of age: consult a doctor.
Inactive Ingredients:
AQUA, PARAFFINUM LIQUIDUM, STEARIC ACID, GLYCERYL MONOSTEARATE, CETYL ALCOHOL, DIMETHICONE, GLYCERETH-26, PROPYLENE GLYCOL, DIAZOLIDINYL UREA, METHYLPARABEN, PROPYLPARABEN, STEARYL ALCOHOL, TRIETHANOLAMINE, ACRYLAMIDE, SODIUM ACRYLATE, TRIDECETH-6, SODIUM HYALURONATE, SODIUM PCA, WHEAT AMINO ACIDS, PANTHENOL, SYMPHYTUM OFFICINALE EXTRACT, HYDROXYPROLINE.