Uses
- cures most athlete's foot (tinea pedis)
- cures most jock itch (tinea cruris) and ringworm (tinea corporis)
- relieves itching, burning, cracking and scaling which accompany these conditions
Warnings
For external use only
Directions
- adults and children 12 years and over:
- use the tip of the cap to break the seal and open the tube
- wash the affected skin with soap and water and dry completely before applying
-
for athlete's foot wear well-fitting, ventilated shoes. Change shoes and socks at least once daily.
- between the toes only: apply twice a day (morning and night) for 1 week or as directed by a doctor
- on the bottom or sides of the foot: apply twice a day (morning and night) for 2 weeks or as directed by a doctor
- for jock itch and ringworm: apply once a day (morning or night) for 1 week or as directed by a doctor
- wash hands after each use
- children under 12 years: ask a doctor
| 1 week between the toes | 2 weeks on the bottom or sides of the foot |
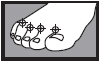 | 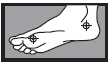 |
Other information
- do not use if seal on tube is broken or is not visible
- store at controlled room temperature 20°-25°C (68°-77°F)
- see carton or tube crimp for lot number and expiration date
Inactive ingredients
benzyl alcohol, cetyl alcohol, cetyl palmitate, isopropyl myristate, polysorbate 60, purified water, sodium hydroxide, sorbitan monostearate, stearyl alcohol.
PRINCIPAL DISPLAY PANEL - 15 g Tube Carton
LEADER™
NDC 70000-0338-1
Full Prescription Strength
Athlete's Foot Cream
Terbinafine Hydrochloride Cream 1%
Antifungal Cream
COMPARE TO
LAMISIL AT®
active ingredient*
100% Money Back Guarantee
Cures Most Athlete's Foot
Relieves Itching, Burning, Cracking and Scaling
NET WT ½ oz (15 g)
