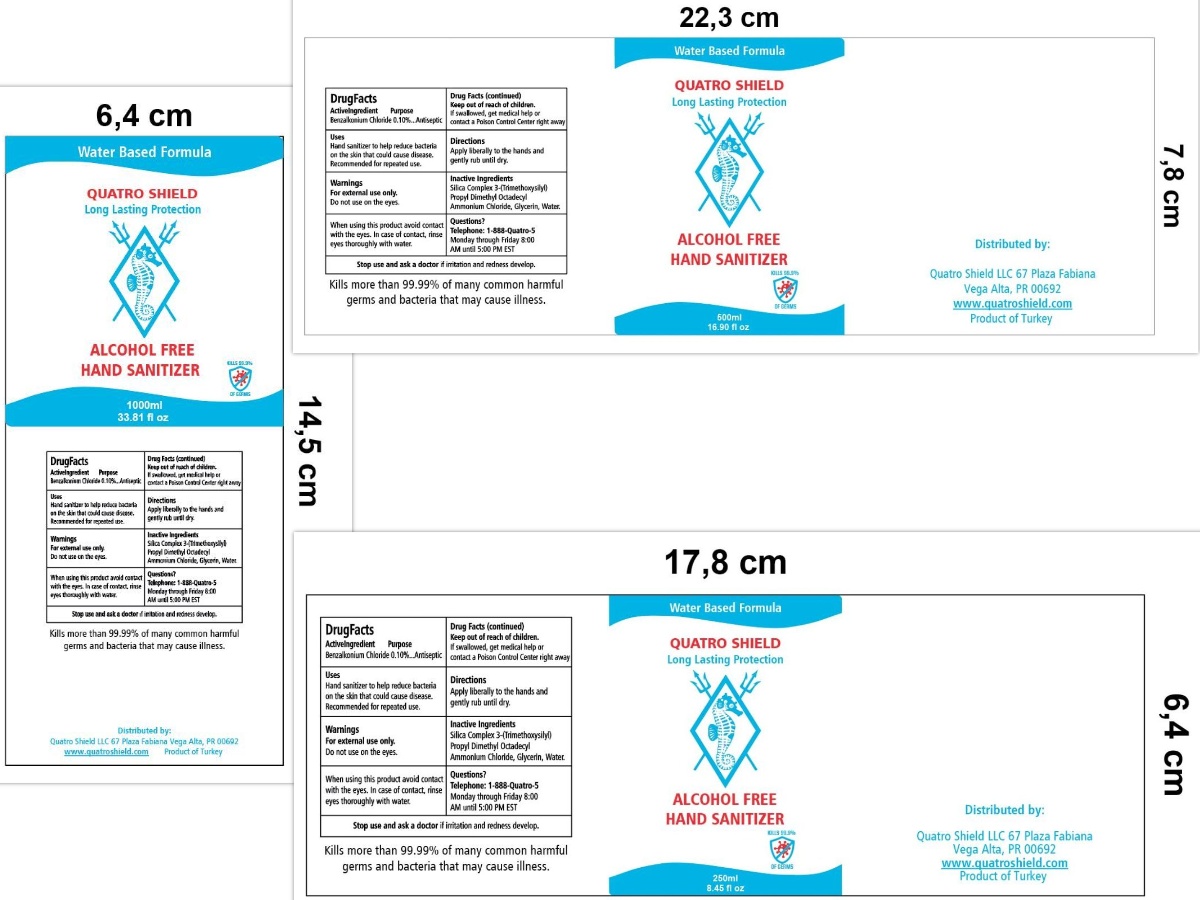
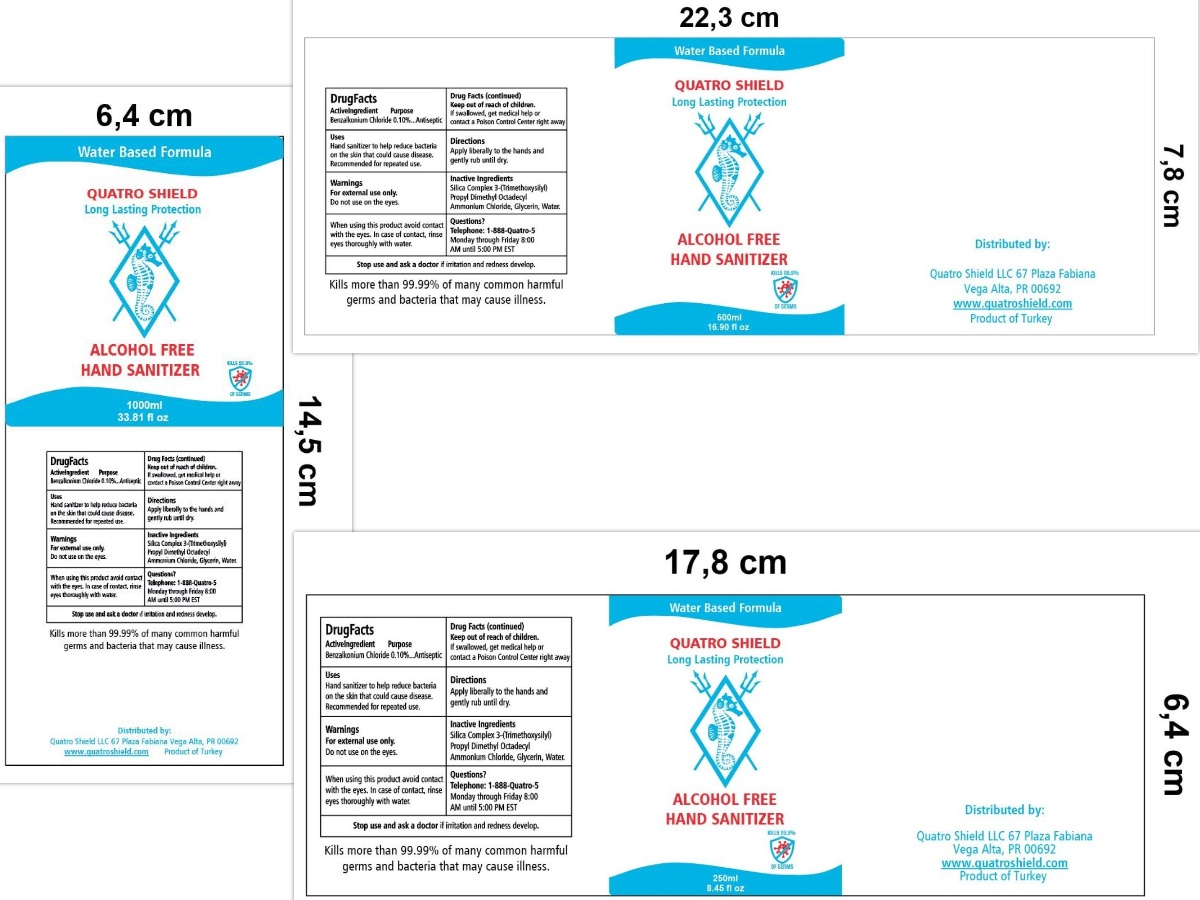
Use
HAND SANITIZER TO HELP REDUCE BACTERIA ON THE SKIN THAT COULD CAUSE DISEASE.
RECOMMENDED FOR REPEATED USE.
WHEN USING THIS PRODUCT AVOID CONTACT WITH THE EYES. IN CASE OF CONTACT, RINSE EYES THOROUGH WITH WATER.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients
SILICA COMPLEX 3-(TRIMETHOXYSILYL) PROPYL DIMETHYL OCTADECYL AMMONIUM CHLORIDE, GLYCERIN, WATER
Package Label - Principal Display Panel
TELEPHONE: 1-888-QUATRO-5
MONDAY THROUGH FRIDAY 8:00 AM UNTIL 5:00 PM EST
WATER BASED FORMULA
QUATRO SHIELD
LONG-LASTING PROTECTION
ALCOHOL-FREE
HAND SANITIZER
KILLS 99.9% OF GERMS
Kills more than 99.99% of many common harmful germs and bacteria that may cause ilnness.
Distributed by:
Quatro Shield LLC
67 Plaza Fabiana
Vega Alta, PR 00692
www.Quatroshield.com
Product of Turkey