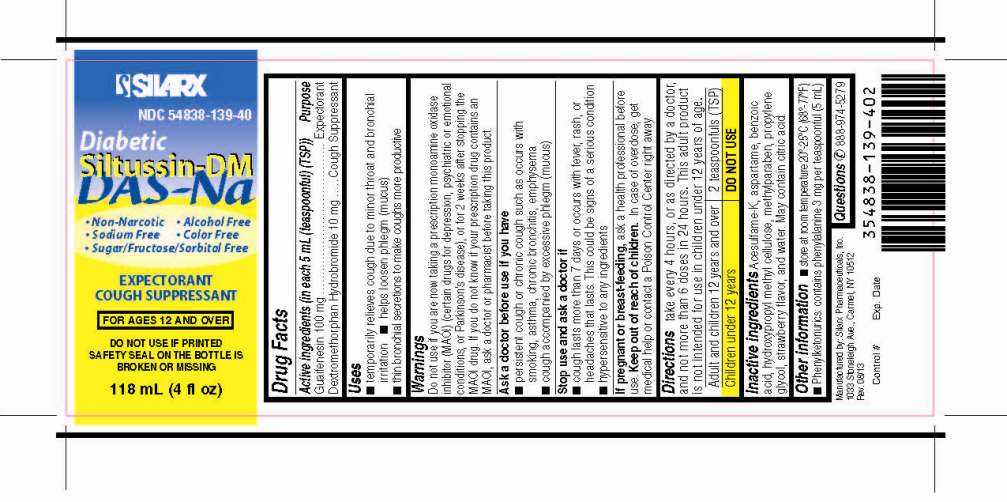
DIABETIC SILTUSSIN DM DAS-NA- guaifenesin and dextromethorphan hydrobromide liquid
Lannett Company, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Diabetic Siltussin DM DAS-Na
Active Ingredient: Guaifenesin 100 mg (in each 5 mL (teaspoonful)(TSP))
Active Ingredient: Dextromethorphan Hydrobromide 10 mg (in each 5 mL (teaspoonful)(TSP))
Uses
■ temporarily relieves cough due to minor throat and bronchial irritation
■ helps loosen phlegm (mucus)
■ thin bronchial secretions to make coughs more productive
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
■ persistent cough or chronic cough such as occurs with smoking, asthma, chronic bronchitis, emphysema
■ cough accompanied by excessive phlegm (mucus)
Directions
take every 4 hours, or as directed by a doctor, and not more than 6 doses in 24 hours. This adult product is not intended for use in children under 12 years of age.
| Adult and children 12 years and over | 2 teaspoonfuls (TSP) |
| Children under 12 years | DO NOT USE |
Other information
■ store at room temperature 20°-25°C (68° - 77°F)
■ Phenylketonurics: contains phenylalanine 3 mg per teaspoonful (5 mL)
Inactive ingredients
Acesulfame-K, aspartame, benzoic acid, hydroxypropyl methyl cellulose, methylparaben, propylene glycol, strawberry flavor, and water. May contain citric acid.
| DIABETIC SILTUSSIN DM DAS-NA
guaifenesin and dextromethorphan hydrobromide liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Lannett Company, Inc. (002277481) |