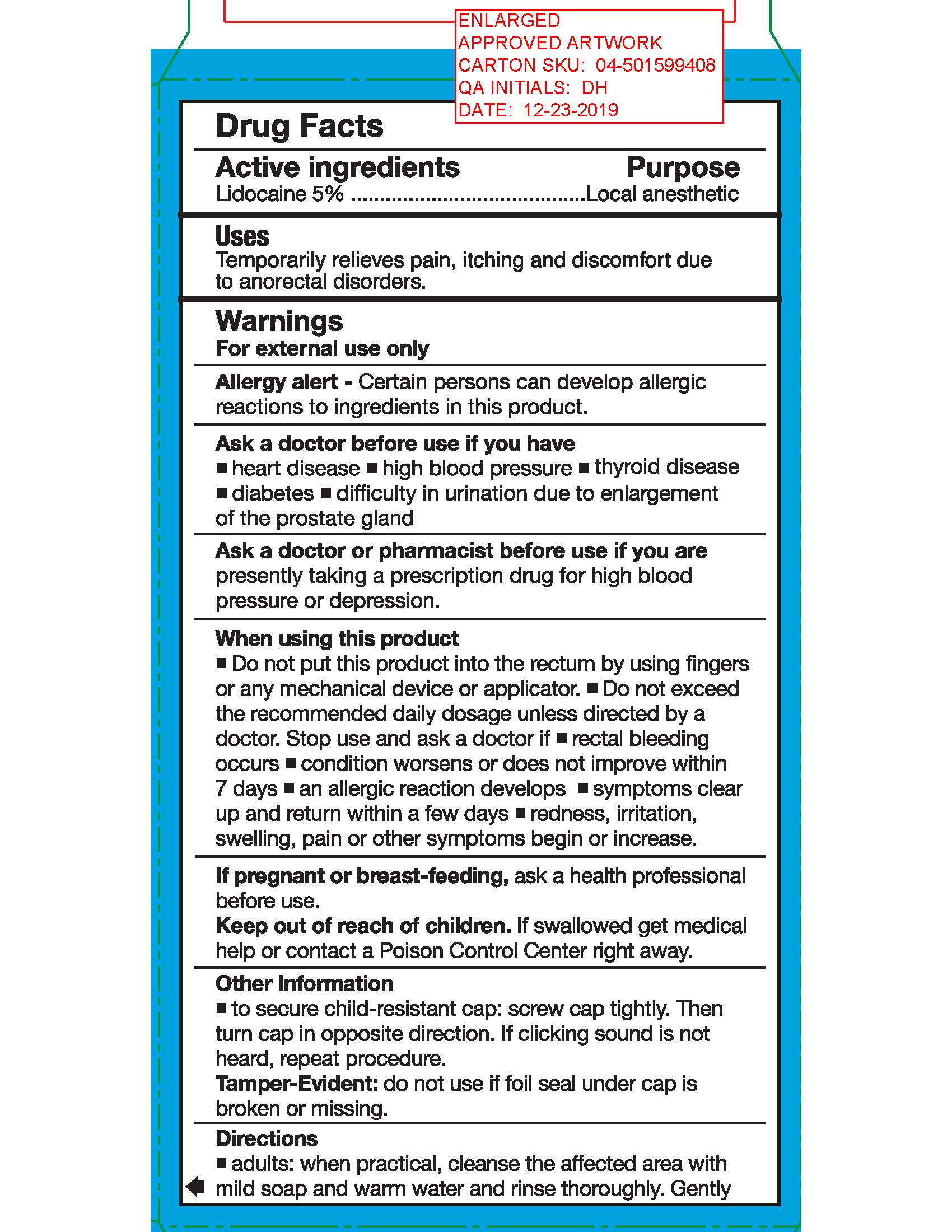
Uses
Temporarily relieves pain, itching and discomfort due to anorectal disorders and temporarily shrinks hemorrhoidal tissue.
Warnings
For External use only
Allergy alert: Certain persons can develop allergic reactions to ingredients in this product
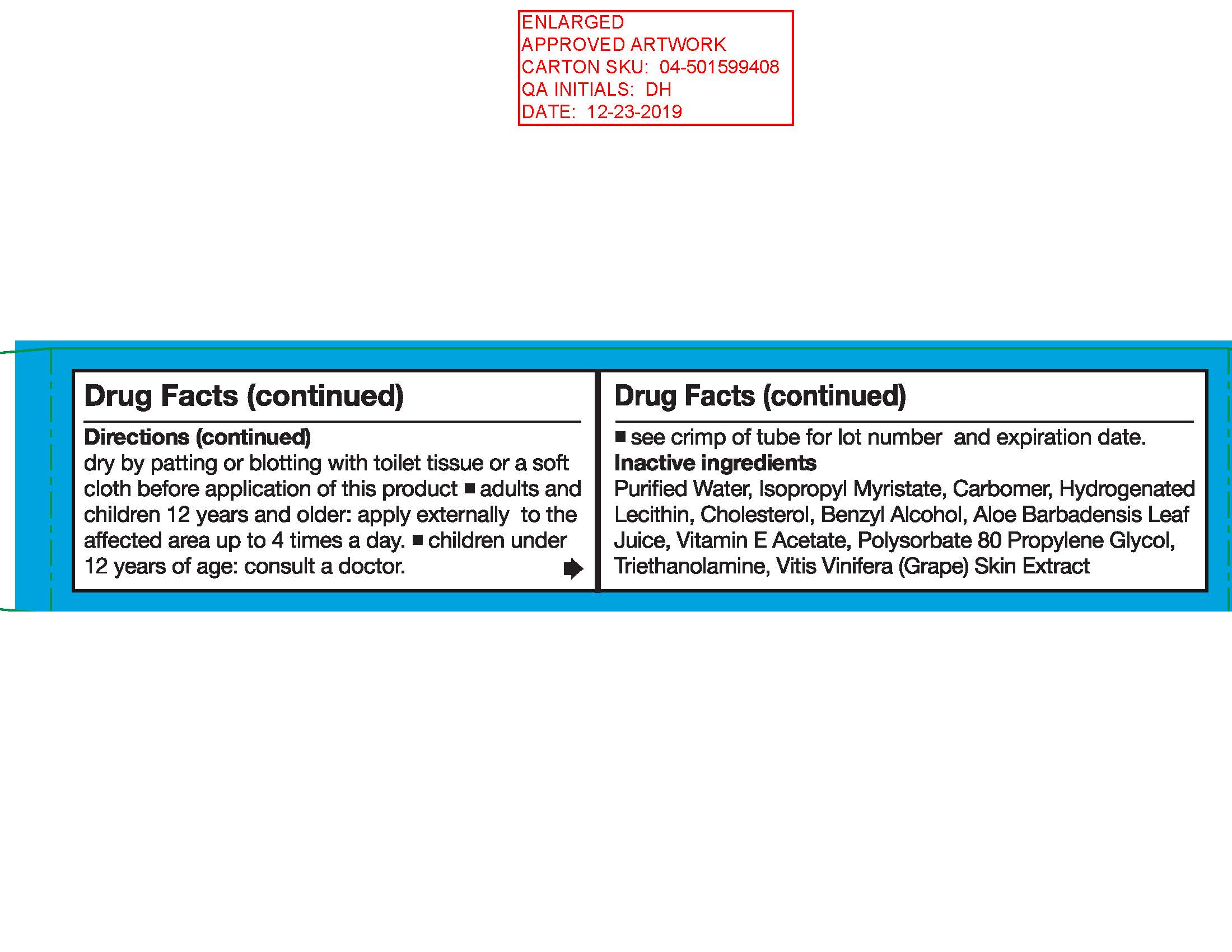
Directions
- Adults: when practical, cleanse the affected area with mild soap and warm water. Rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
- Adults and children 12 years and older: apply externally to the affected area up to 4 times a day.
- Children under 12 years of age: consult a doctor.
Other information
- To secure child-resistant cap: screw cap tightly. Then turn cap in opposite direction. If clicking sound is not heard, repeat procedure.
- Tamper-Evident: do not use if foil seal under cap is broken or missing.
- See crimp of tube for lot number and expiration date.
Inactive ingredients: Aloe Barbadensis Leaf Juice Extract, Benzyl Alcohol, Carbomer, Cholesterol, Hydrogenated Lecithin, Isopropyl Myristate, Polysorbate 80, Propylene Glycol, Purified Water, Triethanolamine, vitamin E Acetate, Vitis Vinifera (Grape) Skin Extract
Keep out of reach of children. If swallowed get medical help or contact a Poison Control Center right away.
Ask a doctor before use if you have
Heart disease
High blood pressure
Thyroid disease
Diabetes
Difficulty in urination due to enlargement of the prostate gland
Stop use and ask a doctor if
Rectal bleeding occurs
Condition worsens or does not improve within 7 days
Allergic reaction occurs
Symptoms clear up and return within a few days
Redness, irritation, swelling, pain or other symptoms begin or increase
Ask a doctor or pharmacist before use if you are presently taking a prescription drug for high blood pressure or depression.
If pregnant or breast-feeding, ask a health professional before use.