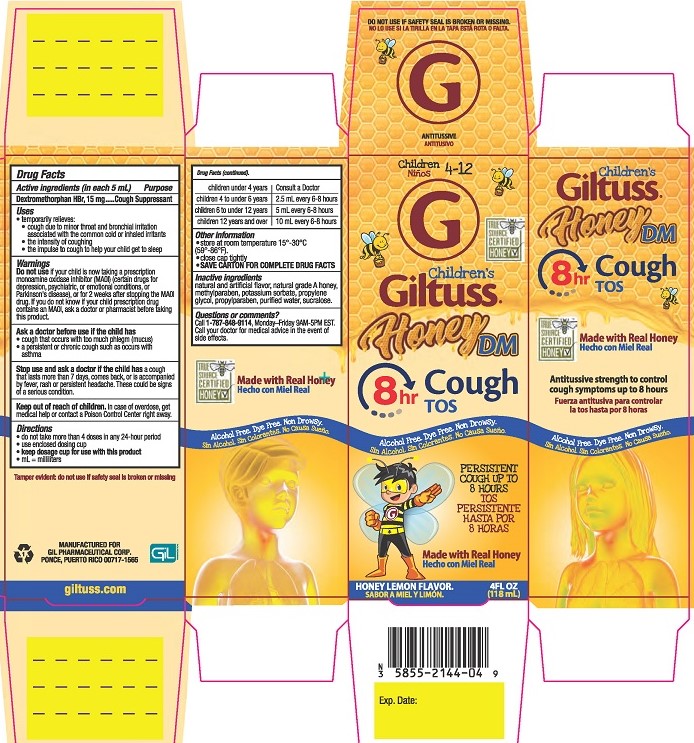
Uses
- temporarily relieves:
- cough due to minor throat and bronchial irritation associated with the common cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help your child get to sleep
Warnings
Do not useif your child is now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if the child has
- cough that occurs with too much phlegm (mucus)
- a persistent or chronic cough such as occurs with asthma
Directions
- do not take more than 4 doses in any 24-hour period
- use enclosed dosing cup
- keep dosage cup for use with this product
- ml = milliliters
| children under 4 years | Consult a doctor |
| children 4 to under 6 years | 2.5 mL every 6-8 hours |
| children 6 to under 12 years | 5 mL every 6-8 hours |
| children 12 years and over | 10 mL every 6-8 hours |
Other information
- store at room temperature 15°-30°C (59°-86°F)
- close cap tightly
- SAVE CARTON FOR COMPLETE DRUG FACTS
Inactive ingredientsnatural and artificial flavor, natural grade A honey, methylparaben, potassium sorbate, propylene glycol, propylparaben, purified water, sucralose.
Questions or comments?Call 1-787-848-9114, Monday-Friday 9AM - 5PM EST. Call your doctor for medical advice in the event of side effects.