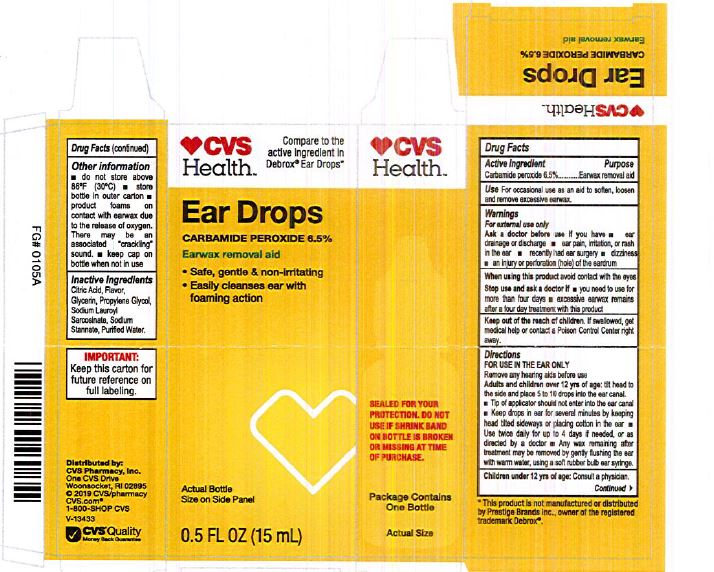
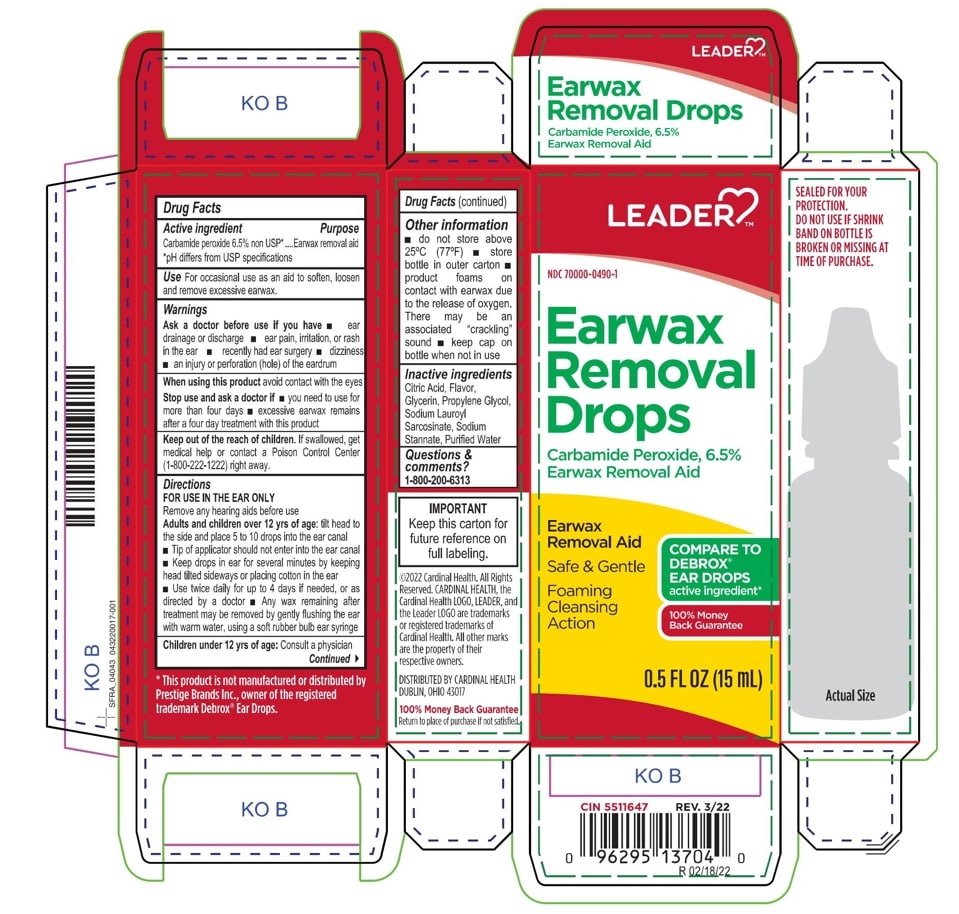
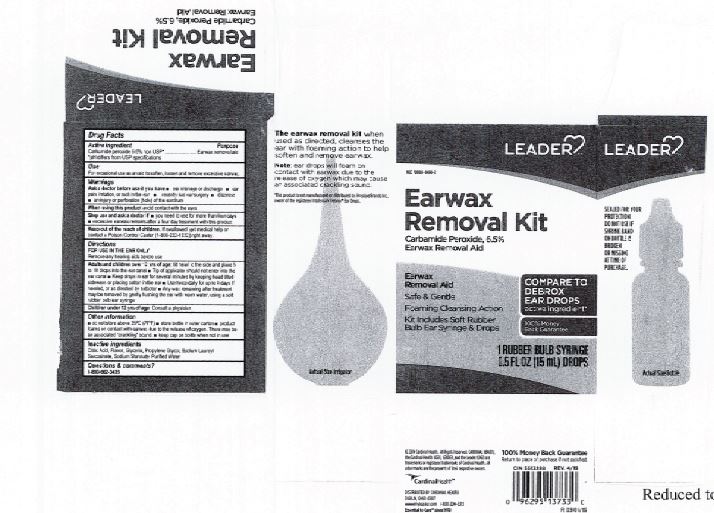
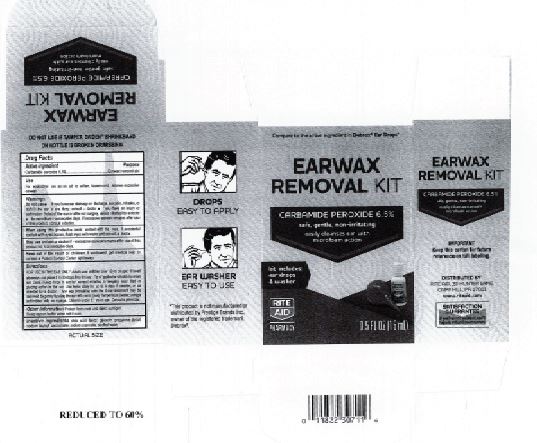
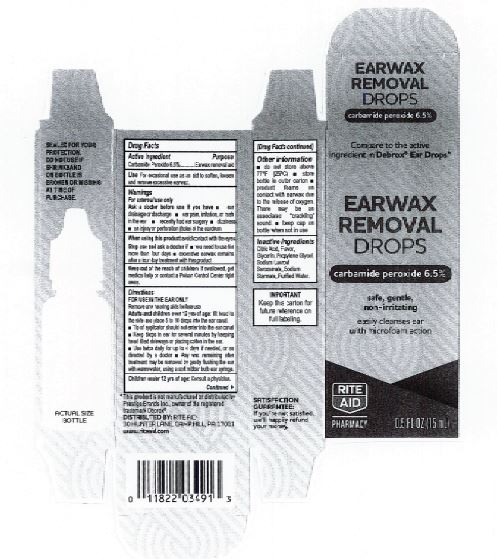
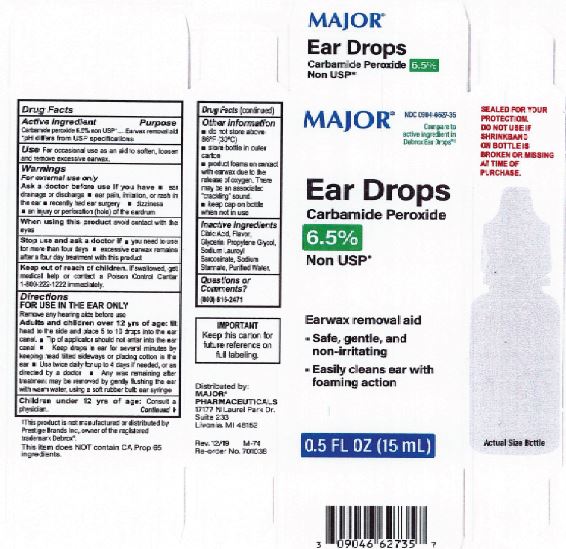
Citric Acid
Flavor
Glycerin
Propylene Glycol
Sodium Lauroyl Sarcosinate
Sodium Stannate
Purified Water
Adults and children over 12 yrs of age: tilt head to the side and place 5 to 10 drops into the ear canal.
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.