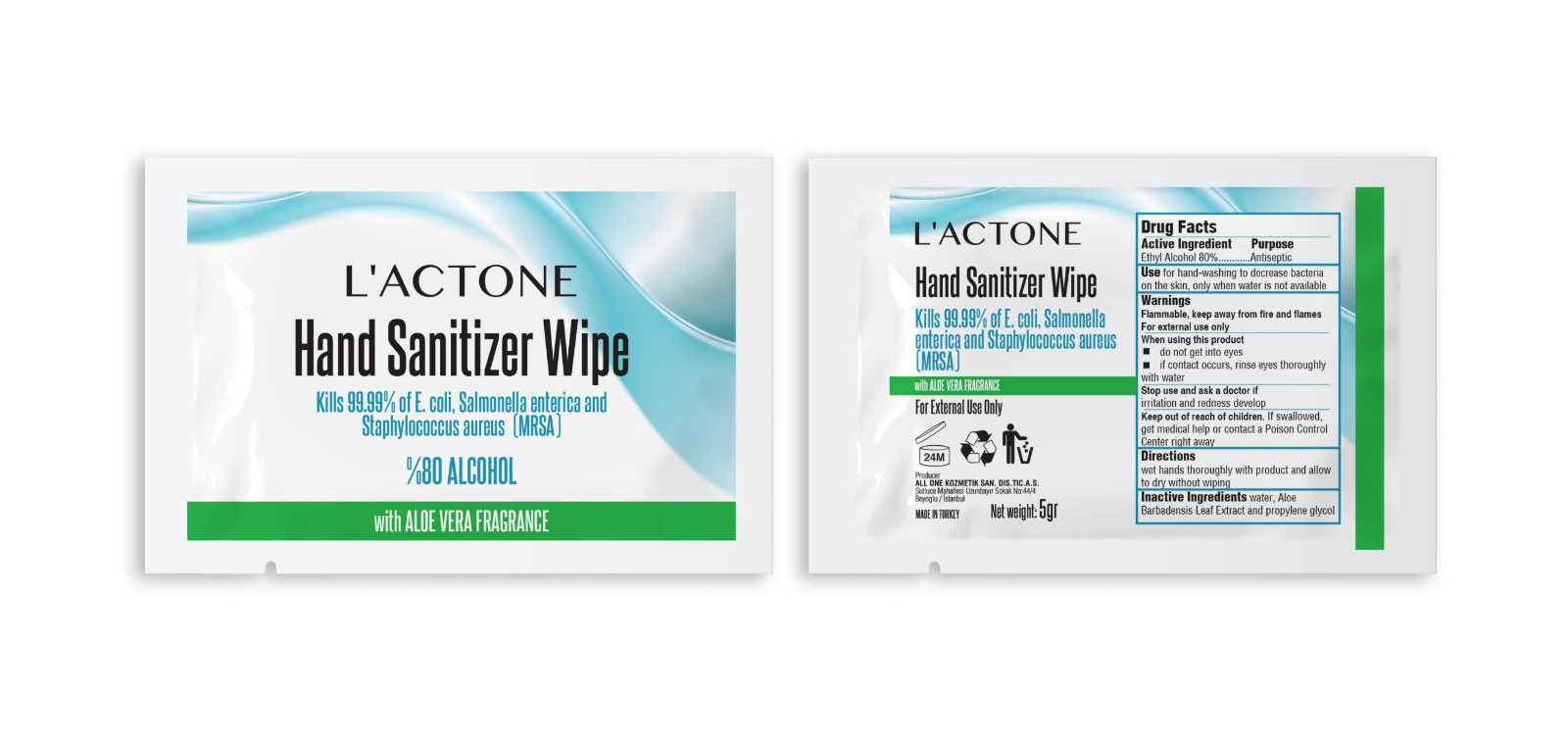
LACTONE HAND SANITIZER WIPE- alcohol cloth
ALL ONE KOZMETIK SANAYI DIS TICARET ANONIM SIRKETI
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient(s)
Ethyl Alcohol 80%
Use
Use for hand-washing to decrease bacteria on the skin, only when water is not available
Warnings
Flammable, keep away from heat and flames. For external use only
When using this product
do not get into eyes
if contact occurs, rinse eyes thoroughly with water
Stop use and ask a doctor if irritation or rash occurs.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
wet hands thorougly with product and allow to dry without wiping
Inactive ingredients
water, Aloe Barbadensis Leaf Extract and propylene glycol
Package Label - Principal Display Panel
Producer
ALL ONE KOZMETIK SAN. VE DIS. TIC. A. S.
Sutluce Mahallesi
Uzunbayır Sokak No:44/4
Beyoglu/Istanbul
Net weight: 5 gr
MADE IN TURKEY
Hand Sanitizer Wipe
Kills 99.99% of E. coli, Salmonella enterica and Staphylococcus aureus (MRSA)
with ALOE VERA FRAGRANCE
For External Use only
LACTONE HAND SANITIZING WIPES- alcohol cloth