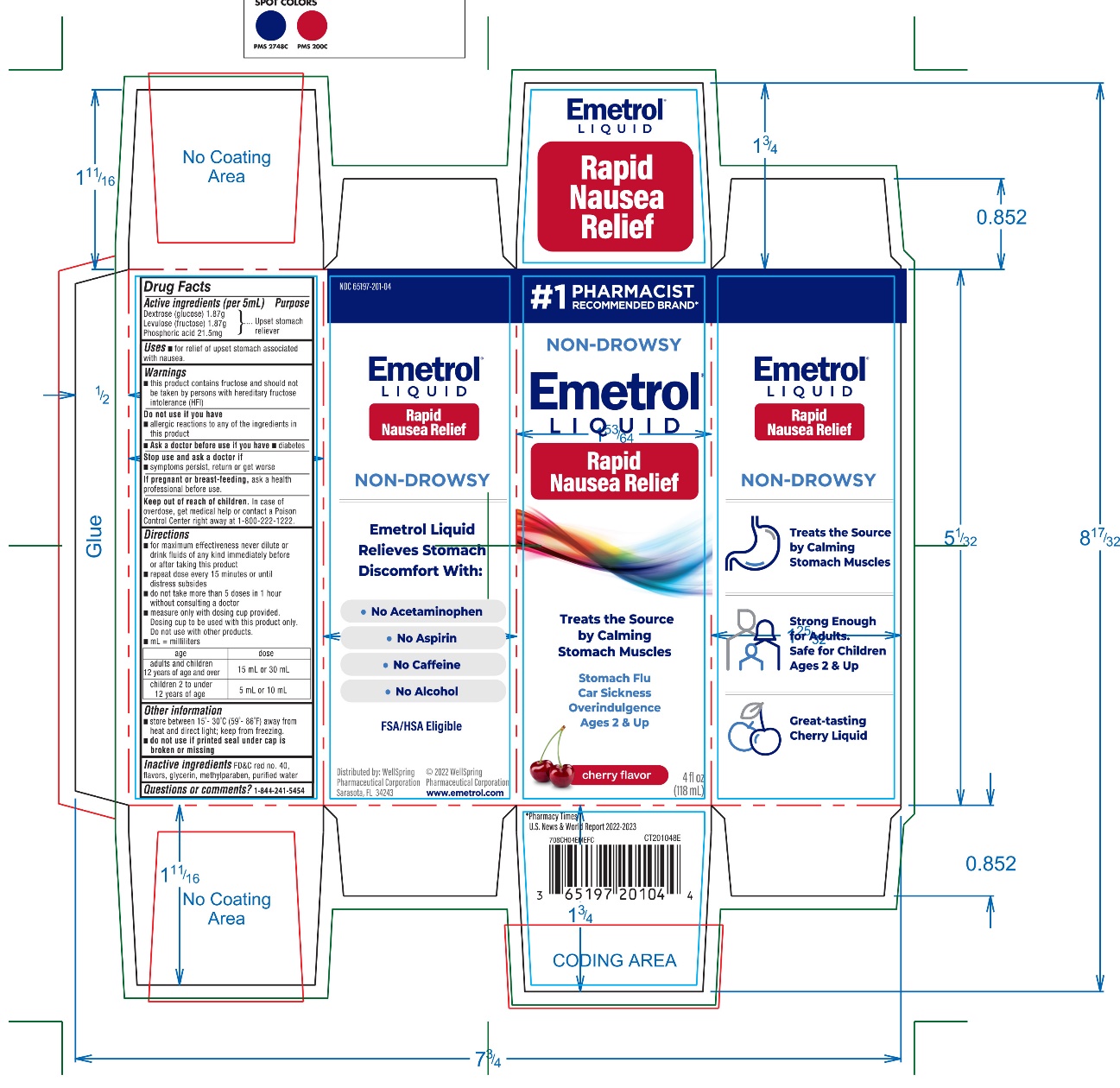
Active ingredients (per 5mL)
Dextrose (glucose) 1.87g
Levulose (fructose) 1.87g
Phosphoric acid 21.5mg
Purpose
Upset Stomach Reliever
Uses
- ▪
- for relief of upset stomach associated with nausea
Warnings
- •
- This product contains fructose and should not be taken by persons with hereditary fructose intolerance (HFI).
Do not use if you have
- •
- allergic reactions to any of the ingredients in this product
Ask a doctor before use if you have
- •
- diabetes
Stop use and ask a doctor if
- •
- symptoms persist, return or get worse
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- ▪
- for maximum effectiveness never dilute or drink fluids of any kind immediately before or after taking this product
- ▪
- repeat dose every 15 minutes or until distress subsides
- ▪
- do not take more than 5 doses in 1 hour without consulting a doctor
- ▪
- measure only with dosing cup provided. Dosing cup to be used with product only. Do not use with other products.
- ▪
- mL = milliliters
|
Age
|
Dose
|
|
Adults and children 12 years of age and over
|
15 mL or 30 mL
|
|
Children 2 to under 12 years of age
|
5 mL or 10 mL
|
Other information
- •
- Store between 15-30ºC (59-86ºF) away from heat and direct light; keep from freezing
- •
-
Do not use if printed foil seal under bottle cap is broken or missing
Inactive ingredients
FD&C red no. 40, flavors, glycerin, methylparaben, and purified water.
Questions or Comments?
call 1-844-241-5454
Distributed By
© 2020 WellSpring Pharmaceutical Corporation
Sarasota, Florida 34243 USA
Made in USA
PACKAGE LABEL