1 INDICATIONS AND USAGE
Mupirocin ointment is indicated for the topical treatment of impetigo due to susceptible isolates of Staphylococcus aureus (S. aureus) and Streptococcus pyogenes (S. pyogenes).
2 DOSAGE AND ADMINISTRATION
- For Topical Use Only.
- Apply a small amount of mupirocin ointment, with a cotton swab or gauze pad, to the affected area 3 times daily for up to 10 days.
- Cover the treated area with gauze dressing if desired.
- Re-evaluate patients not showing a clinical response within 3 to 5 days.
- Mupirocin ointment is not for intranasal, ophthalmic, or other mucosal use [see Warnings and Precautions ( 5.2, 5.6)].
- Do not apply mupirocin ointment concurrently with any other lotions, creams or ointments [see Clinical Pharmacology ( 12.3)].
3 DOSAGE FORMS AND STRENGTHS
- Ointment: Each gram contains 20 mg mupirocin USP in a water-miscible ointment base supplied in 22-gram tubes. ( 3)
4 CONTRAINDICATIONS
- Known hypersensitivity to mupirocin or any of the excipients of mupirocin ointment. ( 4)
Mupirocin ointment is contraindicated in patients with known hypersensitivity to mupirocin or any of the excipients of mupirocin ointment.
5 WARNINGS AND PRECAUTIONS
- Severe Allergic Reactions: Anaphylaxis, urticaria, angioedema, and generalized rash have been reported in patients treated with formulations of mupirocin, including mupirocin ointment. ( 5.1)
- Eye Irritation: Avoid contact with eyes. ( 5.2)
- Local Irritation: Discontinue in the event of sensitization or severe local irritation. ( 5.3)
- Clostridium difficile-Associated Diarrhea (CDAD): If diarrhea occurs, evaluate patients for CDAD. ( 5.4)
- Potential for Microbial Overgrowth: Prolonged use may result in overgrowth of nonsusceptible microorganisms, including fungi. ( 5.5)
- Risk Associated with Mucosal Use: Mupirocin ointment is not formulated for use on mucosal surfaces. A separate formulation, BACTROBAN nasal ointment, is available for intranasal use. ( 5.6)
- Risk of Polyethylene Glycol Absorption: Mupirocin ointment should not be used where absorption of large quantities of polyethylene glycol is possible, especially if there is evidence of moderate or severe renal impairment. ( 5.7)
- Risk Associated with Use at Intravenous Sites: Mupirocin ointment should not be used with intravenous cannulae or at central intravenous sites because of the potential to promote fungal infections and antimicrobial resistance. ( 5.8)
5.1 Severe Allergic Reactions
Systemic allergic reactions, including anaphylaxis, urticaria, angioedema, and generalized rash, have been reported in patients treated with formulations of mupirocin, including mupirocin ointment [see Adverse Reactions ( 6.2)].
5.2 Eye Irritation
Avoid contact with the eyes. In case of accidental contact, rinse well with water.
5.3 Local Irritation
In the event of a sensitization or severe local irritation from mupirocin ointment, usage should be discontinued, and appropriate alternative therapy for the infection instituted.
5.4 Clostridium difficile-Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.5 Potential for Microbial Overgrowth
As with other antibacterial products, prolonged use of mupirocin ointment may result in overgrowth of nonsusceptible microorganisms, including fungi [see Dosage and Administration ( 2)].
5.6 Risk Associated with Mucosal Use
Mupirocin ointment is not formulated for use on mucosal surfaces. Intranasal use has been associated with isolated reports of stinging and drying. A separate formulation, †Bactroban (mupirocin calcium) nasal ointment, is available for intranasal use.
5.7 Risk of Polyethylene Glycol Absorption
Polyethylene glycol can be absorbed from open wounds and damaged skin and is excreted by the kidneys. In common with other polyethylene glycol-based ointments, mupirocin ointment should not be used in conditions where absorption of large quantities of polyethylene glycol is possible, especially if there is evidence of moderate or severe renal impairment.
6 ADVERSE REACTIONS
- The most frequent adverse reactions (at least 1%) were burning, stinging or pain, and itching. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Glenmark Pharmaceuticals Inc., USA at 1-888-721-7115 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Severe Allergic Reactions [see Warnings and Precautions ( 5.1)]
- Eye Irritation [see Warnings and Precautions ( 5.2)]
- Local Irritation [see Warnings and Precautions ( 5.3)]
- Clostridium difficile-Associated Diarrhea [see Warnings and Precautions ( 5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following local adverse reactions were reported by at least 1% of subjects in connection with the use of mupirocin ointment in clinical trials: burning, stinging, or pain in 1.5% of subjects; itching in 1% of subjects. Rash, nausea, erythema, dry skin, tenderness, swelling, contact dermatitis, and increased exudate were reported in less than 1% of subjects.
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following reactions have been identified during postmarketing use of mupirocin ointment. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal relationship to mupirocin ointment.
Immune System Disorders
Systemic allergic reactions, including anaphylaxis, urticaria, angioedema, and generalized rash [see Warnings and Precautions ( 5.1)].
8 USE IN SPECIFIC POPULATIONS
Click here to enter Use in Specific Populations
8.1 Pregnancy
Risk Summary
There are insufficient human data to establish whether there is a drug-associated risk with mupirocin ointment in pregnant women. Systemic absorption of mupirocin through intact human skin is minimal following topical administration of mupirocin ointment [see Clinical Pharmacology ( 12.3)] . No developmental toxicity was observed in rats or rabbits treated with mupirocin subcutaneously during organogenesis at doses of 160 or 40 mg per kg per day, respectively (22 and 11 times the human topical dose based on calculations of dose divided by the entire body surface area).
The estimated background risk of major birth defects and miscarriages for the indicated population is unknown. The estimated background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Animal Data: Developmental toxicity studies have been performed with mupirocin administered subcutaneously to rats and rabbits at doses up to 160 mg per kg per day during organogenesis. This dose is 22 and 43 times, respectively, the human topical dose (approximately 60 mg mupirocin per day) based on calculations of dose divided by the entire body surface area. Maternal toxicity was observed (body weight loss/decreased body weight gain and reduced feeding) in both species with no evidence of developmental toxicity in rats. In rabbits, excessive maternal toxicity at the high dose precluded the evaluation of fetal outcomes. There was no developmental toxicity in rabbits at 40 mg per kg per day, 11 times the human topical dose based on calculations of dose divided by the entire body surface area.
Mupirocin administered subcutaneously to rats in a pre-and postnatal development study (dosed during late gestation through lactation) was associated with reduced offspring viability in the early postnatal period at a dose of 106.7 mg per kg, in the presence of injection site irritation and/or subcutaneous hemorrhaging. This dose is 14 times the human topical dose based on calculations of dose divided by the entire body surface area. The no-observed adverse effect level in this study was 44.2 mg per kg per day, which is 6 times the human topical dose.
8.2 Lactation
Risk Summary
It is not known whether mupirocin is present in human milk, has effects on the breastfed child, or has effects on milk production. However, breastfeeding is not expected to result in exposure of the child to the drug due to the minimal systemic absorption of mupirocin in humans following topical administration of mupirocin ointment [see Clinical Pharmacology ( 12.3)] . The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for mupirocin ointment and any potential adverse effects on the breastfed child from mupirocin ointment or from the underlying maternal condition.
Clinical Considerations
To minimize oral exposure of the drug to children, a breast and/or nipple being treated with mupirocin ointment should be thoroughly washed prior to breastfeeding.
8.4 Pediatric Use
The safety and effectiveness of mupirocin ointment have been established in the age range of 2 months to 16 years. Use of mupirocin ointment in these age-groups is supported by evidence from adequate and well-controlled trials of mupirocin ointment in impetigo in pediatric subjects studied as a part of the pivotal clinical trials [see Clinical Studies ( 14)].
11 DESCRIPTION
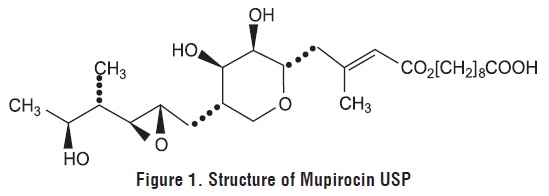
Mupirocin ointment USP 2% contains the RNA synthetase inhibitor antibacterial, mupirocin USP. The chemical name is ( E)-(2 S,3 R,4 R,5 S)-5-[(2 S,3 S,4 S,5 S)-2,3-epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4dihydroxy-ß-methyl-2 H-pyran-2-crotonic acid, ester with 9-hydroxynonanoic acid. The molecular formula of mupirocin USP is C 26H 44O 9, and the molecular weight is 500.6. The chemical structure is:
Each gram of mupirocin ointment USP 2% contains 20 mg mupirocin USP in a water-miscible ointment base (polyethylene glycol ointment, N.F.) consisting of polyethylene glycol 400 and polyethylene glycol 3350.
12 CLINICAL PHARMACOLOGY
Click here to enter Clinical Pharmacology
12.1 Mechanism of Action
Mupirocin is an RNA synthetase inhibitor antibacterial [see Microbiology ( 12.4)].
12.3 Pharmacokinetics
Absorption
Application of 14C-labeled mupirocin ointment to the lower arm of normal male subjects followed by occlusion for 24 hours showed no measurable systemic absorption (less than 1.1 nanogram mupirocin per milliliter of whole blood). Measurable radioactivity was present in the stratum corneum of these subjects 72 hours after application.
The effect of the concurrent application of mupirocin ointment with other drug products has not been studied [see Dosage and Administration ( 2)].
Elimination
In a trial conducted in 7 healthy adult male subjects, the elimination half-life after intravenous administration of mupirocin was 20 to 40 minutes for mupirocin and 30 to 80 minutes for monic acid.
Metabolism: Following intravenous or oral administration, mupirocin is rapidly metabolized. The principal metabolite, monic acid, demonstrates no antibacterial activity.
Excretion: Monic acid is predominantly eliminated by renal excretion.
12.4 Microbiology
Mupirocin is an RNA synthetase inhibitor antibacterial produced by fermentation using the organism Pseudomonas fluorescens.
Mechanism of Action
Mupirocin inhibits bacterial protein synthesis by reversibly and specifically binding to bacterial isoleucyltransfer RNA (tRNA) synthetase.
Mupirocin is bactericidal at concentrations achieved by topical administration. Mupirocin is highly protein bound (greater than 97%) and the effect of wound secretions on the minimum inhibitory concentrations (MICs) of mupirocin has not been determined.
Mechanism of Resistance
When mupirocin resistance occurs, it results from the production of a modified isoleucyl-tRNA synthetase, or the acquisition of, by genetic transfer, a plasmid mediating a new isoleucyl-tRNA synthetase. High-level plasmid-mediated resistance (MIC =512 mcg/mL) has been reported in increasing numbers of isolates of S. aureus and with higher frequency in coagulase-negative staphylococci. Mupirocin resistance occurs with greater frequency in methicillin-resistant than methicillin-susceptible staphylococci.
Cross Resistance
Due to its mode of action, mupirocin does not demonstrate cross resistance with other classes of antimicrobial agents.
Antimicrobial Activity
Mupirocin has been shown to be active against susceptible isolates of S. aureus and S. pyogenes, both in vitro and in clinical trials [see Indications and Usage ( 1)]. The following in vitro data are available, but their clinical significance is unknown. Mupirocin is active against most isolates of Staphylococcus epidermidis.
Susceptibility Testing
High-level mupirocin resistance (=512 mcg/mL) may be determined using standard disk diffusion or broth microdilution tests. 1,2 Because of the occurrence of mupirocin resistance in methicillin-resistant S. aureus (MRSA), it is appropriate to test MRSA populations for mupirocin susceptibility prior to the use of mupirocin using a standardized method. 3,4,5
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential of mupirocin have not been conducted.
Results of the following studies performed with mupirocin calcium or mupirocin sodium in vitro and in vivo did not indicate a potential for genotoxicity: rat primary hepatocyte unscheduled DNA synthesis, sediment analysis for DNA strand breaks, Salmonella reversion test (Ames), Escherichia coli mutation assay, metaphase analysis of human lymphocytes, mouse lymphoma assay, and bone marrow micronuclei assay in mice.
In a fertility/reproductive performance study (with dosing through lactation), mupirocin administered subcutaneously to male and female rats at doses up to 100 mg per kg per day which is 14 times the human topical dose (approximately 60 mg mupirocin per day) based on calculations of dose divided by the entire body surface area, did not result in impaired fertility or impaired reproductive performance attributable to mupirocin.
14 CLINICAL STUDIES
The efficacy of topical mupirocin ointment in impetigo was tested in 2 trials. In the first, subjects with impetigo were randomized to receive either mupirocin ointment or vehicle placebo 3 times daily for 8 to 12 days. Clinical efficacy rates at end of therapy in the evaluable populations (adults and pediatric subjects included) were 71% for mupirocin ointment (n = 49) and 35% for vehicle placebo (n = 51). Pathogen eradication rates in the evaluable populations were 94% for mupirocin ointment and 62% for vehicle placebo.
In the second trial, subjects with impetigo were randomized to receive either mupirocin ointment 3 times daily or 30 to 40 mg per kg oral erythromycin ethylsuccinate per day (this was an unblinded trial) for 8 days. There was a follow-up visit 1 week after treatment ended. Clinical efficacy rates at the follow-up visit in the evaluable populations (adults and pediatric subjects included) were 93% for mupirocin ointment (n = 29) and 78.5% for erythromycin (n = 28). Pathogen eradication rates in the evaluable populations were 100% for both test groups.
Pediatrics
There were 91 pediatric subjects aged 2 months to 15 years in the first trial described above. Clinical efficacy rates at end of therapy in the evaluable populations were 78% for mupirocin ointment (n = 42) and 36% for vehicle placebo (n = 49). In the second trial described above, all subjects were pediatric except 2 adults in the group receiving mupirocin ointment. The age range of the pediatric subjects was 7 months to 13 years. The clinical efficacy rate for mupirocin ointment (n = 27) was 96%, and for erythromycin it was unchanged (78.5%).
15 REFERENCES
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-fifth Informational Supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, 950 West Valley Rd., Suite 2500, Wayne, PA 19087, USA, 2015.
- Patel J, Gorwitz RJ, et al. Mupirocin Resistance. Clinical Infectious Diseases. 2009; 49(6): 935-41.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Tenth Edition. CLSI document M07-A10. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standard – Twelfth Edition. CLSI document M02-A12. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
- Finlay JE, Miller LA, Poupard JA. Interpretive criteria for testing susceptibility of staphylococci to mupirocin. Antimicrob Agents Chemother 1997;41(5):1137-1139.
16 HOW SUPPLIED/STORAGE AND HANDLING
Mupirocin ointment USP 2% is supplied in 22-gram tubes.
Each gram of mupirocin ointment USP contains 20 mg mupirocin USP in a water-miscible ointment base.
NDC 68462-180-22 22-gram tube (1 tube per carton)
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling ( Patient Information).
Advise the patient to administer mupirocin ointment as follows:
- Use mupirocin ointment only as directed by the healthcare provider. It is for external use only. Avoid contact of mupirocin ointment with the eyes. If mupirocin ointment gets in the eyes, rinse thoroughly with water.
- Do not use mupirocin ointment in the nose.
- Wash your hands before and after applying mupirocin ointment.
- Use a gauze pad or cotton swab to apply a small amount of mupirocin ointment to the affected area. The treated area may be covered by gauze dressing if desired.
- Report to the healthcare provider any signs of local adverse reactions. Mupirocin ointment should be stopped and the healthcare provider contacted if irritation, severe itching, or rash occurs.
- Report to the healthcare provider or go to the nearest emergency room if severe allergic reactions, such as swelling of the lips, face, or tongue, or wheezing occur [see Warnings and Precautions ( 5.1)].
- If impetigo has not improved in 3 to 5 days, contact the healthcare provider.
†Bactroban is a registered trademark of the GSK group of companies.
Manufactured by:
Glenmark Pharmaceuticals Ltd.
Colvale-Bardez, Goa 403 513, India
Manufactured for:
Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888)721-7115
www.glenmarkpharma.com/usa
December 2015
Patient Information
Mupirocin (mue-PIR-oh-sin)
Ointment USP
What is mupirocin ointment?
Mupirocin ointment is a prescription medicine used on the skin (topical use) to treat a skin infection called impetigo that is caused by bacteria called Staphylococcus aureus and Streptococcus pyogenes. It is not known if mupirocin ointment is safe and effective in children under 2 months of age.
Who should not use mupirocin ointment?
Do not use mupirocin ointment if:
- you are allergic to mupirocin or any of the ingredients in mupirocin ointment. See the end of this Patient Information leaflet for a complete list of the ingredients in mupirocin ointment.
What should I tell my healthcare provider before using mupirocin ointment?
Before using mupirocin ointment, tell your healthcare provider about all of your medical conditions including if you:
- have kidney problems
- are pregnant or plan to become pregnant. It is not known if mupirocin ointment will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if mupirocin ointment passes into your breast milk. You and your healthcare provider should decide if you can use mupirocin ointment while breastfeeding.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Do not mix mupirocin ointment with other lotions, creams, or ointments.
How should I use mupirocin ointment?
- Mupirocin ointment is for use on the skin (topical). Do not get mupirocin ointment in your eyes, nose, mouth, or vagina (mucosal surfaces).
- Use mupirocin ointment exactly as your healthcare provider tells you to use it.
- Apply a small amount of mupirocin ointment, with a cotton swab or gauze pad, to the affected area 3 times each day.
- It is important that you take the full course of mupirocin ointment. Do not stop early because your symptoms may disappear before the infection is fully cleared.
- Wash your hands before and after applying mupirocin ointment.
- After applying mupirocin ointment, you may cover the treated area with a clean gauze pad, unless your healthcare provider has told you to leave it uncovered.
- Talk to your healthcare provider if your skin does not improve after 3 to 5 days of treatment with mupirocin ointment.
What are the possible side effects of mupirocin ointment?
Mupirocin ointment may cause serious side effects, including:
- severe allergic reactions. Stop using mupirocin ointment and call your healthcare provider or go to the nearest emergency room right away if you have any of the following signs or symptoms of a severe allergic reaction:
- hives
- trouble breathing or wheezing
- swelling of your face, lips, mouth, or tongue
- dizziness, fast heartbeat, or pounding in your chest
- a rash over your whole body
- eye irritation. Do not get mupirocin ointment in your eyes. If mupirocin ointment gets in your eyes, rinse your eyes well with water.
- irritation in the area mupirocin ointment is used. Stop using mupirocin ointment and call your healthcare provider if you develop an irritation, severe itching, or a rash while using mupirocin ointment.
- a type of diarrhea called clostridium difficile-associated diarrhea (CDAD). CDAD may happen in people who use or have used medicine to treat bacterial infections. The severity of CDAD can range from mild diarrhea to severe diarrhea that may cause death (fatal colitis). Call your healthcare provider or go to the nearest emergency room right away if you have diarrhea while using or after you stop using mupirocin ointment.
- risk of absorption of polyethylene glycol through the skin. Mupirocin ointment contains polyethylene glycol, which in large amounts can cause kidney damage. You should not apply mupirocin ointment to open skin wounds or damaged skin, especially if you have kidney problems.
- increased risk of infection at IV (intravenous) sites. Mupirocin ointment should not be used on skin that is near an IV (intravenous) site
The most common side effects of mupirocin ointment include:
- burning
- stinging or pain
- itching
These are not all the possible side effects of mupirocin ointment. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store mupirocin ointment?
- Store at 20° to 25°C (68° to 77°F).
Keep mupirocin ointment and all medicines out of the reach of children.
General information about the safe and effective use of mupirocin ointment
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use mupirocin ointment for a condition for which it was not prescribed. Do not give mupirocin ointment to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about mupirocin ointment that is written for health professionals.
What are the ingredients in mupirocin ointment?
Active Ingredient: mupirocin USP
Inactive Ingredients: polyethylene glycol 400 and polyethylene glycol 3350
†Bactroban is a registered trademark of the GSK group of companies.
Manufactured by:
Glenmark Pharmaceuticals Ltd.
Colvale-Bardez, Goa 403 513, India
Manufactured for:
Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888)721-7115
www.glenmarkpharma.com/usa
December 2015
USES
- surgical hand scrub: significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care
- healthcare personnel handwash: helps reduce bacteria that potentially can cause disease
- patient preoperative skin preparation: for the preparation of the patient's skin prior to surgeryskin wound and general skin cleansing
- skin wound and general skin cleansing
WARNINGS
For external use only.
Allergy alert:
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
DO NOT USE
- if you are allergic to chlorhexidine gluconate or any other ingredients
- in contact with meninges
- in the genital area
- as a preoperative skin preparation of the head or face
WHEN USING THIS PRODUCT
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if placed or kept in the eye during surgical procedures or may cause deafness when instilled in the middle ear through perforated eardrums.
- if solution should contact these areas, rinse out promptly and thoroughly with water
- wounds which involve more than the superficial layers of the skin should not be routinely treated
- repeated general skin cleansing of large body areas should not be done except when advised by a health care provider
DIRECTION
- use with care in premature infants and infants under 2 months of age. These products may cause irritation or chemical burns.
Surgical hand scrub:
- wet hands and forearms with water
- scrub for 3 minutes with about 5 ml of product and a wet brush paying close attention to the nails, cuticles and interdigital spaces
- a separate nail cleaner may be used
- rinse thoroughly
- wash for an additional 3 minutes with 5 ml of product and rinse under running water
- dry thoroughly
Healthcare personnel handwash:
- wet hands with water
- dispense about 5 ml of product into cupped hands and wash in a vigorous manner for 15 seconds
- rinse and dry thoroughly
Patient preoperative skin preparation:
- apply product liberally to surgical site and swab for at least 2 minutes and dry with a sterile towel
- repeat procedure for an additional 2 minutes and dry with a sterile towel
Skin wound and general skin cleaning:
- thoroughly rinse the area to be cleaned with water
- apply the minimum amount of product necessary to cover the skin or wound area and wash gently
- rinse again thoroughly
INACTIVE INGREDIENTS
cocamide DEA, fragrance, glucono-delta-lactone, hydroxyethylcellulose, isopropyl alcohol, lauramine oxide, PEG-75 lanolin, purified water, tridecyl alcohol
INDICATIONS & USAGE
Uses
For the treatment and / or prevention of diaper rash temporarily and helps relieve chapped or cracked skin
WARNINGS
For external use only.
- When using this product
- do not get into eyes
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
STOP USE
Stop use and ask a doctor if
Condition worsens
symptoms last more than 7 days or clear up occur again within a few days
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away
DOSAGE & ADMINISTRATION
Directions
- Cleanse skin with Thera Moisturizing Body Cleanser or Thera Foaming Body Cleanser
- Apply cream liberally until entire area is covered
- Apply as needed
INACTIVE INGREDIENTS
Inactive Ingredients:
Purified Water, Glyceryl Stearate, PEG 100 Stearate, Stearic Acid, Aleurites Moluccana Seed Oil,
Cetyl Alcohol, Glycerin, Butylene Glycol, Carthamus Tinctorius (Safflower) Seed Oil,
Dimethicone Crosspolymer, Calcium Pantothenate (Vitamin B5), Maltodextrin, Niacinamide (Vitamin B3),
Pyridoxine HCL (Vitamin B6), Silica, Sodium Ascorbyl Phosphate (Vitamin C), Sodium Starch Octenylsuccinate,
Tocopheryl Acetate (Vitamin E), Disodium EDTA, Sodium Hyaluronate, Aloe Barbadensis Leaf Extract,
Zingiber Officinale (Ginger) Root Extract, Carthamus Tinctorius (Safflower) Oleosomes, Fragrance,
Phenoxyethanol, Caprylyl Glycol, Chlorphenesin, Bisabolol, Lysine, Histidine, Arginine, Aspartic Acid,
Threonine, Serine, Glutamic Acid, Proline, Glycine, Alanine, Valine, Methionine, Isoleucine, Leucine,
Tyrosine, Phenylalanine, Cysteine, Triethanolamine