Uses
- temporarily relieves pain and itching associated with:
- insect bites
- minor burns
- sunburn
- minor skin irritations
- minor cuts
- scrapes
- rashes due to poison ivy, poison oak, and poison sumac
- dries the oozing and weeping of poison ivy, poison oak and poison sumac
Warnings
For external use only.
Flammable. Keep away from fire or flame.
Do not use
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
Directions
- do not use more than directed
- adults and children 2 years of age and older: spray on affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
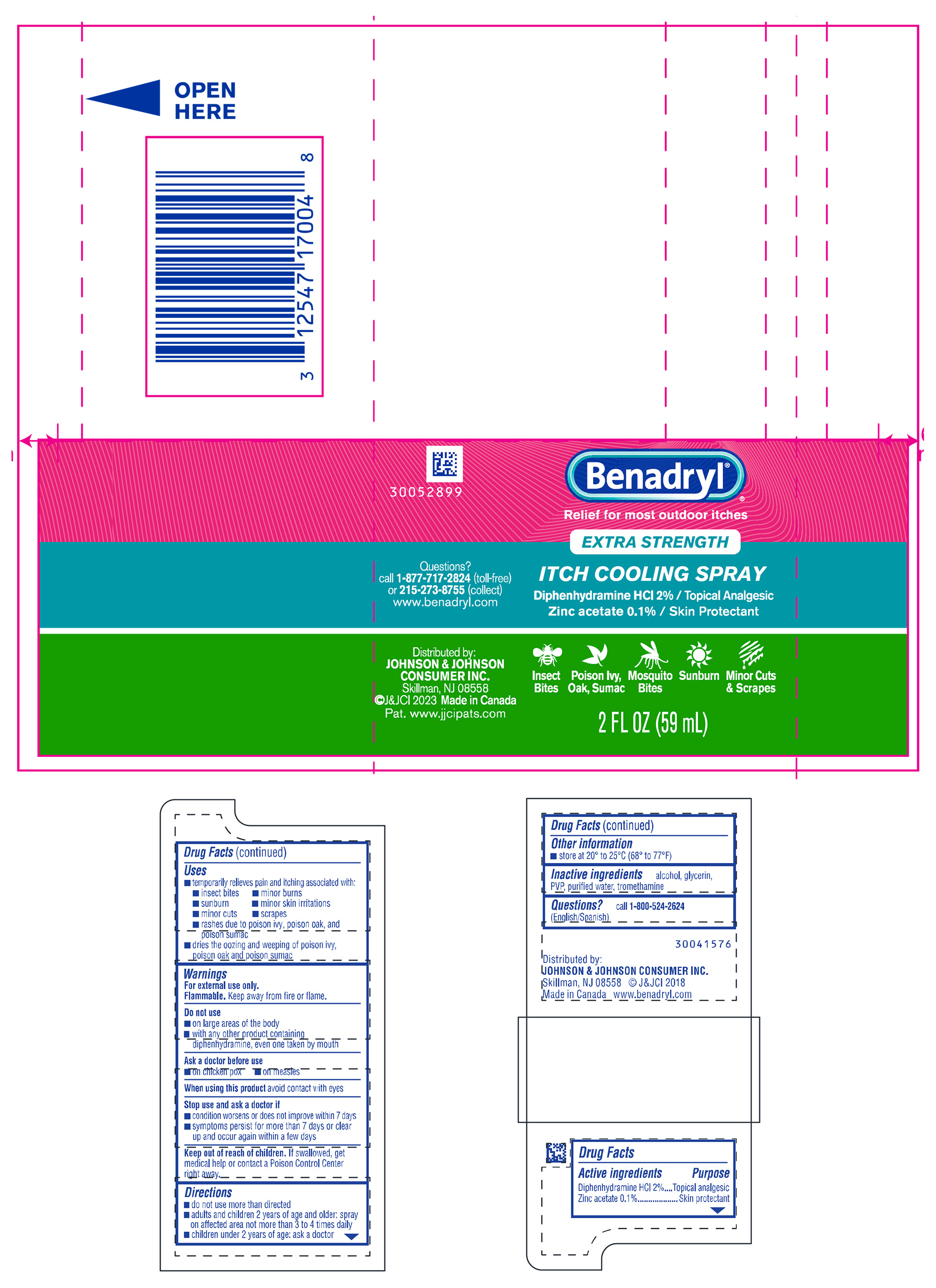
PRINCIPAL DISPLAY PANEL - 59 mL Bottle Label
Benadryl®
Relief for most outdoor itches
EXTRA STRENGTH
ITCH COOLING SPRAY
Diphenhydramine HCl 2% / Topical Analgesic
Zinc acetate 0.1 % / Skin Protectant
Questions?
call 1-877-717-2824 (toll-free)
or 215-273-8755 (collect)
www.benadryl.com
| Insect
Bites | Poison Ivy,
Oak, Sumac | Mosquito
Bites | Sunburn | Minor Cuts
& Scrapes |
Distributed by:
JOHNSON & JOHNSON
CONSUMER INC.
Skillman, NJ 08558
J&JCI 2023 Made in Canada
Pat.www.jjcipats.com
2 FL OZ (59 mL)