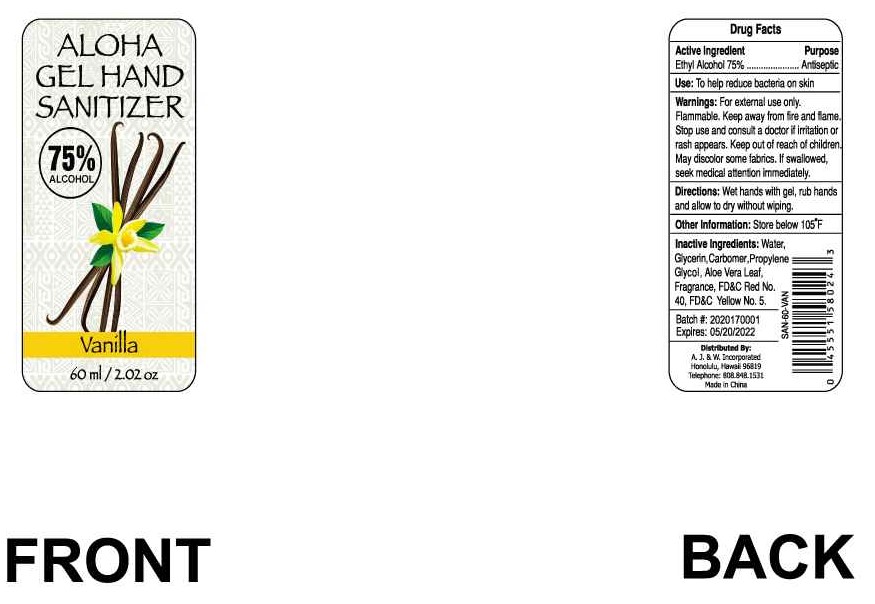
Uses
Hand Sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
Inactive Ingredient:
(only for Package NDC: 74657-010-01; 74657-010-05; 74657-010-10)*
Water, Glycerin, Carbomer, Propylene Glycol, Aloe Barbadensis Leaf Extract.
(only for Package NDC: 74657-011-01)*
Water, Glycerin, Carbomer, Propylene Glycol, Aloe Vera Leaf, Frangrance, FD&C Red No. 40, FD&C Yellow No. 5.
(only for Package NDC: 74657-012-01)*
Water, Glycerin, Carbomer, Propylene Glycol, Aloe Vera Leaf, Frangrance, FD&C Red No. 33.
(only for Package NDC: 74657-013-01)*
Water, Glycerin, Carbomer, Propylene Glycol, Aloe Vera Leaf, Frangrance, FD&C Red 40, FD&C Yellow No. 5.
(only for Package NDC: 74657-014-01)*
Water, Glycerin, Carbomer, Propylene Glycol, Aloe Vera Leaf, Frangrance, FD&C Blue No. 1, FD&C Yellow No. 5.
(only for Package NDC: 74657-015-01)*
Water, Glycerin, Carbomer, Propylene Glycol, Aloe Vera Leaf, Frangrance
*Remark: , All words in the brackets are not shown on the labels.