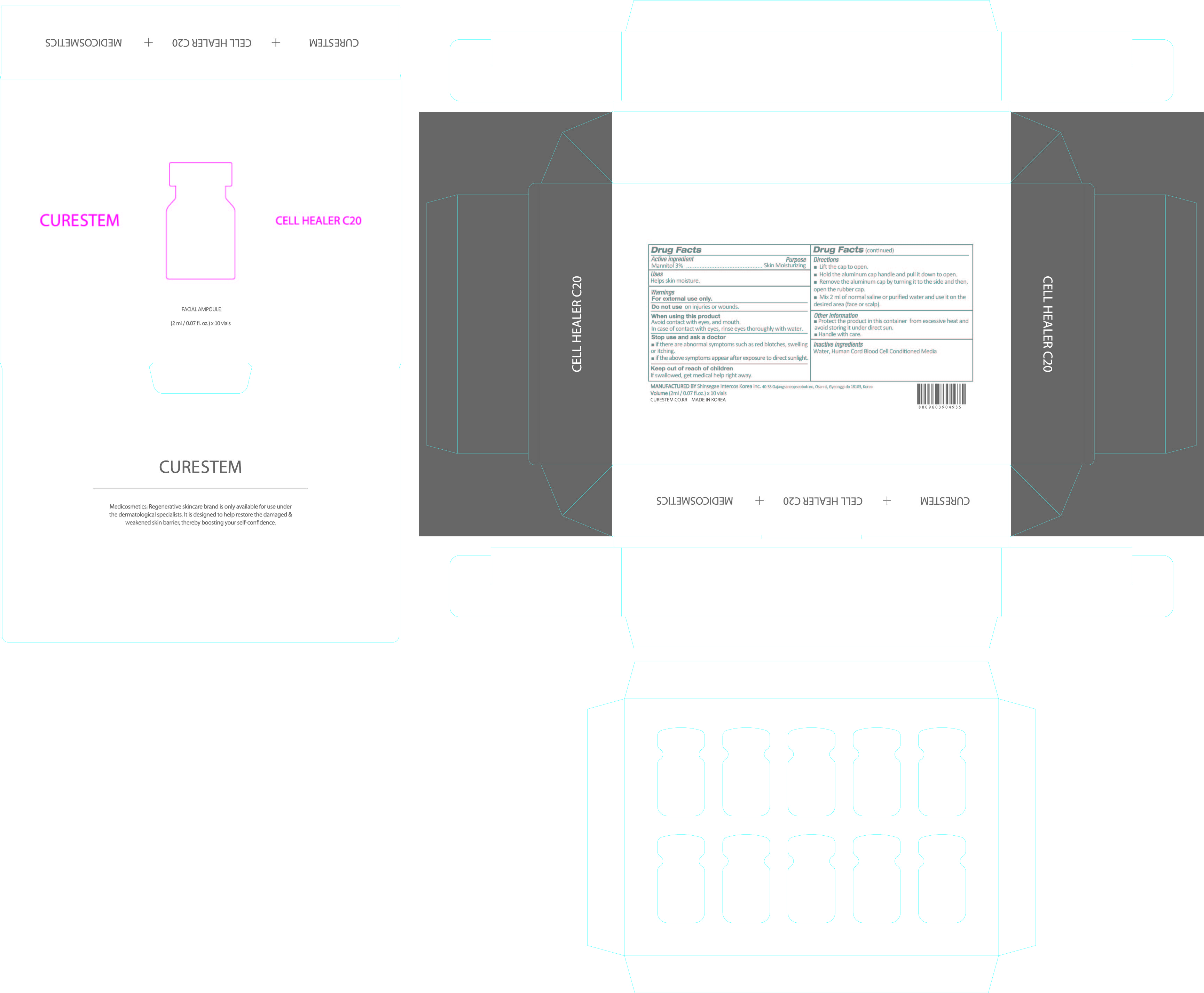
When using this product
Avoid contact with eyes, and mouth.
In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor
■ if there are abnormal symptoms such as red blotches, swelling or itching.
■ if the above symptoms appear after exposure to direct sunlight.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
■ Lift the cap to open.
■ Hold the aluminum cap handle and pull it down to open.
■ Remove the aluminum cap by turning it to the side and then,
open the rubber cap.
■ Mix 2 ml of normal saline or purified water and use it on the
desired area (face or scalp).