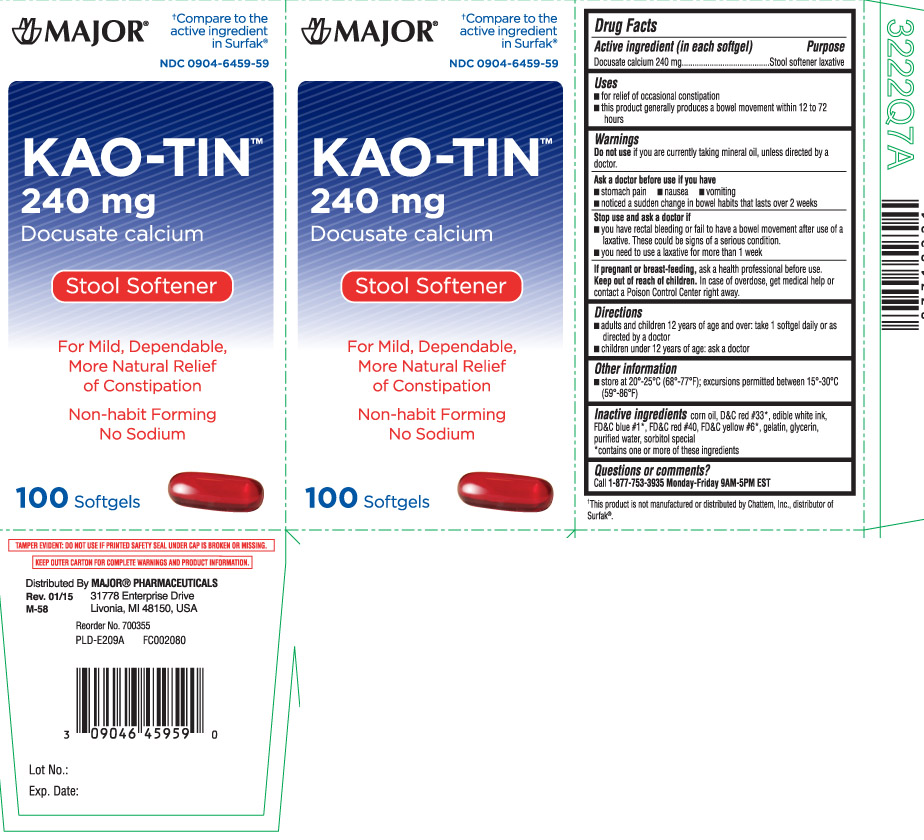
Uses
- for relief of occasional constipation
- this product generally produces a bowel movement within 12 to 72 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Directions
- adults and children 12 years of age and over: take 1 softgel daily or as directed by a doctor
- children under 12 years of age: ask a doctor
Inactive ingredients
corn oil, D&C red #33*, edible white ink, FD&C blue #1, FD&C red #40, FD&C yellow #6, gelatin, glycerin, purified water, sorbitol special
*contains one or more of these ingredients
Principal Display Panel
†Compare to the active ingredient in Surfak®
Kao-Tin
240 mg
Docusate calcium
Stool Softener
For mild, dependable, more natural relief of constipation
Non-habit forming
No sodium
Softgels
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY DEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by: MAJOR PHARMACEUTICALS
31778 Enterorise Drive
Livonia, MI 48150 USA
†This product is not manufactured or distributed by Chattem, Inc., distributor of Surfak®