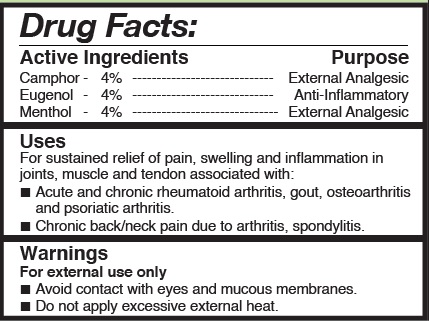
TotalArthritis Active Ingredients
| Camphor | 4% | External Analgesic |
| Eugenol | 4% | Anti-Inflammatory |
| Menthol | 4% | External Analgesic |

Uses
For sustained relief of pain, swelling and Inflammation in joints, muscle and tendon associated with:
• Acute and chronic rheumatoid arthritis, gout, osteoarthritis and psoriatic arthritis.
• Chronic back/neck pain due to arthritis, spondylitis.

Warnings for external use only
- Avoid contact with eyes and mucous membranes.
- Do not apply excessive external heat.
- Use only as directed.
When using this product
- Do not apply excessive external heat
- Avoid contact with eyes and mucous membranes.
Directions
- Apply a generous amount of the ointment (~1g) on affected area 3 times daily and massage gently. For faster results, apply
local heat with a heat lamp or heat pad after each application.
- For chronic severe conditions, use the ointment for 6 to 9 months and as advised by the physician*.
* Individual results vary depending on the chronic conditions, age and time length of suffering.
Other information
- Store in room temperature 40 0F to 86 0F (4 0C to 30 0C)
- Bioactive formula has a natural color that can stain cloths


