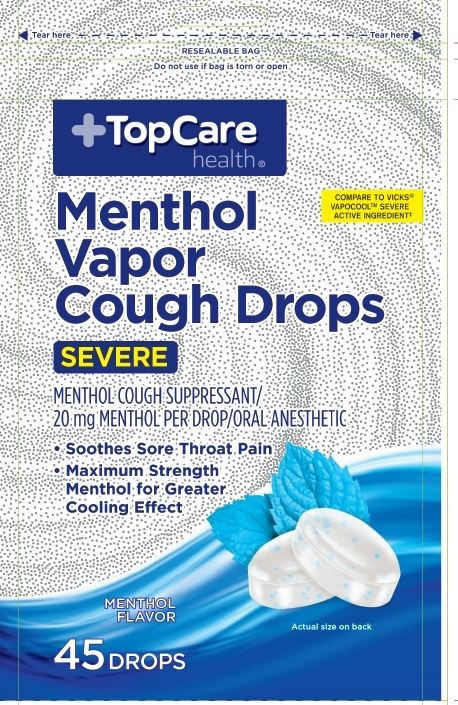
MENTHOL VAPOR COUGH DROPS- menthol pastille
BOSTON NUTRACEUTICAL PRODUCTION, S.L
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient in each drop Purposes
Menthol 20 mg
Severe
Menthol Caugh Suppressant
Oral Anesthetic
Purposes
Temporarily relieves ocassional minor irritation and pain due to:
Purposes
Temporarily relieves ocassional minor irritation and pain due to:
Warnings
Sore throat warning: severe or persistent throat accompanied by high fever headache, nausea and vomiting maybe serious. Consult a doctor promptly. Do not use more than 2 days or administer to children under 12 years of old.
Ask a doctor before use if you have
- A severe throat accompanied by difficulty in breathing or that last more than 2 days
- A severe throat accompanied by fever, headache, rash, swealling, nausea or vomiting.
Stop use and consult doctor if
- sore mouth symptons does not improve in 7 days or if irritation, pain or redness persists or worse
If pregnant or breast feeding, ask a health professional before use.
If pregnant or breast-feeding, ask a health professional before use.
Keep this and all drugs out of the reach of children.
In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 12 years and over: dissolve 1 drop slowly in mouth. Repeated every two hours as needed or as directed by a doctor.
- Children 12 years or under do not use
Other information
- Store in a cool and dry place
corn syrup, sucrose, water, eucalyptus oil, acacia, FD&Blue No.1

Questions or comments call: 1-888-423-0139