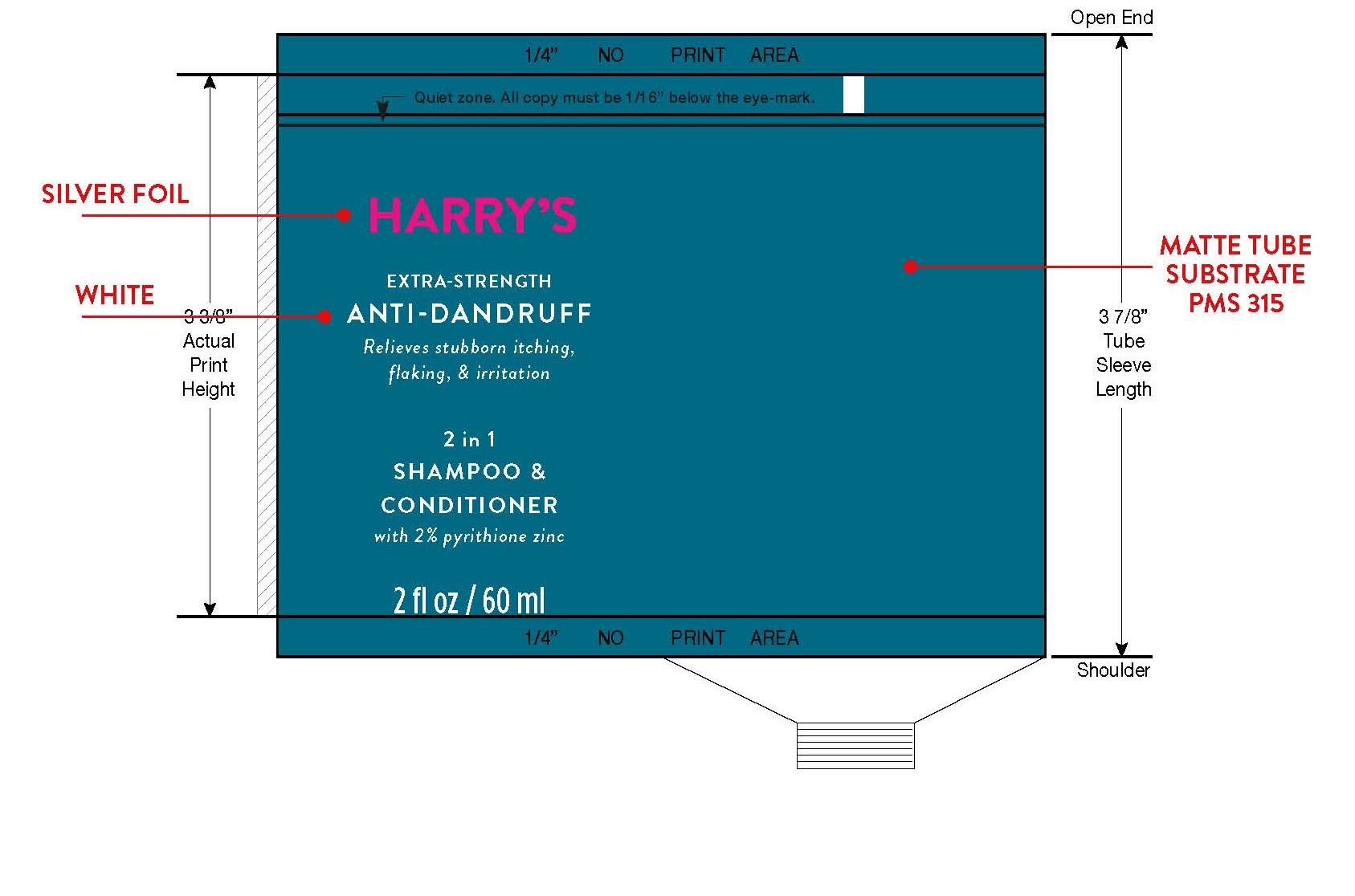
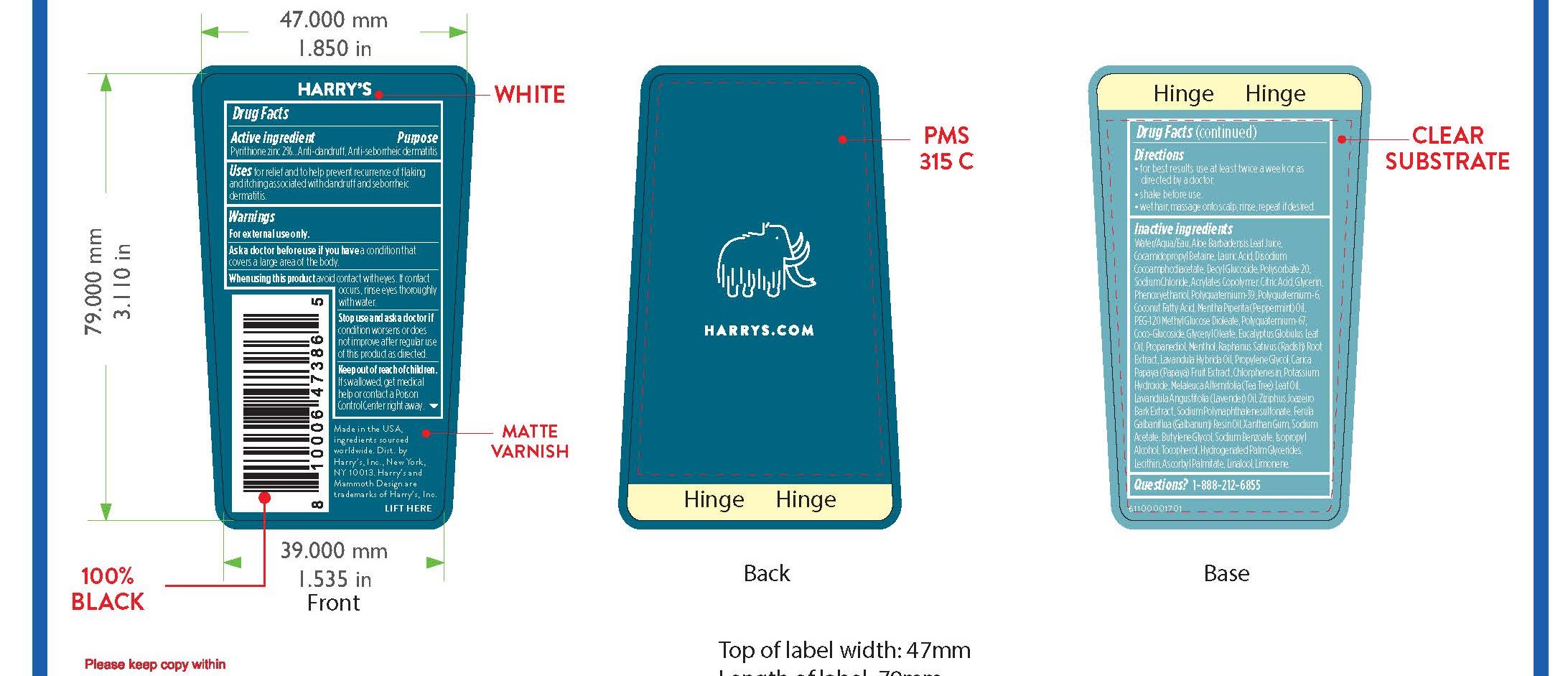
Uses
for relief and to help prevent recurrence of flaking and itching associated with dandruff and seborrheic dermatitis.
Warnings
For external use only.
When using this product avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
• for best results use at least twice a week or as
directed by a doctor.
• shake before use.
• wet hair, massage onto scalp, rinse, repeat if desired.
Inactive ingredients
Water/Aqua/Eau, Aloe Barbadensis Leaf Juice,Cocamidopropyl Betaine, Lauric Acid, Disodium Cocoamphodiacetate, Decyl Glucoside, Polysorbate 20, Sodium Chloride, Acrylates Copolymer, Citric Acid, Glycerin, Phenoxyethanol, Polyquaternium-39, Polyquaternium-6, Coconut Acid, Mentha Piperita (Peppermint) Oil, PEG-120 Methyl Glucose Dioleate, Polyquaternium-67, Coco-Glucoside, Glyceryl Oleate, Eucalyptus Globulus Leaf Oil, Propanediol, Menthol, Raphanus Sativus (Radish) Root Extract, Lavandula Hybrida Oil, Propylene Glycol, Carica Papaya (Papaya) Fruit Extract, Chlorphenesin, Potassium Hydroxide, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Lavandula Angustifolia (Lavender) Oil, Ziziphus Joazeiro Bark Extract, Sodium Polynaphthalenesulfonate, Ferula Galbaniflua (Galbanum) Resin Oil, Xanthan Gum, Sodium Acetate, Butylene Glycol, Sodium Benzoate, Isopropyl Alcohol, Tocopherol, Hydrogenated Palm Glycerides, Lecithin, Ascorbyl Palmitate, Linalool, Limonene.