FULL PRESCRIBING INFORMATION
2 DOSAGE AND ADMINISTRATION
2.1 Indicator-Dilution Studies
In the performance of dye dilution curves, a known amount of dye is injected as a single bolus as rapidly as possible via a cardiac catheter into selected sites in the vascular system. A recording instrument (oximeter or densitometer) is attached to a needle or catheter for sampling of the dye‑blood mixture from a systemic arterial sampling site.
Under sterile conditions, the Indocyanine Green for injection powder should be dissolved with the Sterile Water for injection provided for this product, and the solution used within 6 hours after it is prepared. If a precipitate is present, discard the solution.
The usual doses of Indocyanine Green for injection for dilution curves are as follows:
Adults - 5.0 mg
Children - 2.5 mg
Infants - 1.25 mg
These doses of the dye are usually injected in 1 mL volume. An average of five dilution curves are recommended in the performance of a diagnostic cardiac catheterization. The total dose of dye injected should be kept below 2 mg/kg.
While sterile water for injection may be used to rinse the syringe, isotonic saline should be used to flush the residual dye from the cardiac catheter into the circulation so as to avoid hemolysis. With the exception of the rinsing of the dye injection syringe, saline should be used in all other parts of the catheterization procedure.
Calibrating Dye Curves: To quantitate the dilution curves, standard dilutions of Indocyanine Green for injection in whole blood are made as follows. It is strongly recommended that the same dye that was used for the injections be used in the preparation of these standard dilutions. The most concentrated dye solution is made by accurately diluting 1 mL of the 5 mg/mL dye with 7 mL of distilled water. This concentration is then successively halved by diluting 4 mL of the previous concentration with 4 mL of distilled water.
If a 2.5 mg/mL concentration was used for the dilution curves, 1 mL of the 2.5 mg/mL dye is added to 3 mL of distilled water to make the most concentrated “standard” solution. This concentration is then successively halved by diluting 2 mL of the previous concentration with 2 mL of distilled water.
Then 0.2 mL portions (accurately measured from a calibrated syringe) of these dye solutions are added to 5 mL aliquots of the subject's blood, giving final concentrations of the dye in blood beginning with 24.0 mg/liter, approximately (actual concentration depends on the exact volume of dye added). This concentration is, of course, successively halved in the succeeding aliquots of the subject's blood. These aliquots of blood containing known amounts of dye, as well as a blank sample to which 0.2 mL of saline containing no dye has been added, are then passed through the detecting instrument and a calibration curve is constructed from the deflections recorded.
2.2 Hepatic Function Studies
Due to its absorption spectrum, changing concentrations of Indocyanine Green for injection in the blood can be monitored by ear densitometry or by obtaining blood specimens at timed intervals. The technique for both methods is as follows.
The patient should be studied in a fasting, basal state. The patient should be weighed and the dosage calculated on the basis of 0.5 mg/kg of body weight.
Under sterile conditions, the Indocyanine Green for injection powder should be dissolved with the Sterile Water for injection provided. Exactly 5 mL of Sterile Water for injection should be added to the 25 mg vial giving 5 mg of dye per mL of solution.
Inject the calculated amount of dye (0.5 mg/kg of body weight) into the lumen of an arm vein as rapidly as possible, without allowing the dye to escape outside the vein. (If the photometric method is used, prior to injecting Indocyanine Green for injection, withdraw 6 mL of venous blood from the patient's arm for serum blank and standard curve construction, and through the same needle, inject the correct amount of dye.)
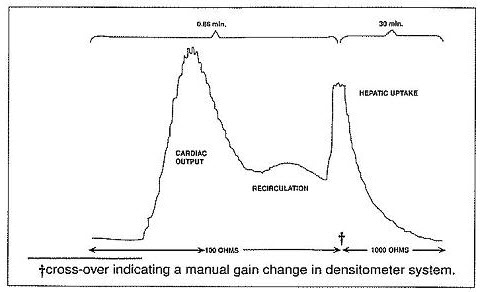
Ear Densitometry: Ear oximetry has also been used and makes it possible to monitor the appearance and disappearance of Indocyanine Green for injection without the necessity of withdrawal and spectrophotometric analysis of blood samples for calibration. An ear densitometer which has a compensatory photo-electric cell to correct for changes in blood volume and hematocrit, and a detection photo cell which registers levels should be used. This device permits simultaneous measurement of cardiac output, blood volume and hepatic clearance of Indocyanine Green for injection*. This technique has been employed in newborn infants, healthy adults and in children and adults with liver disease. The normal subject has a removal rate of 18 to 24% per minute. Due to the absence of extra-hepatic removal, Indocyanine Green for injection was found to be suited for serial study of severe chronic liver disease and to provide a stable measurement of hepatic blood flow. In larger doses, Indocyanine Green for injection can be used in detecting drug-induced alterations of hepatic function and in the detection of mild liver injury.
Using the ear densitometer, a dosage of 0.5 mg/kg in normal subjects gives the following clearance pattern.
*Dichromatic earpiece densitometer supplied by The Waters Company, Rochester, Minnesota.
Photometric Method -
Determination Using Percentage Retention of Dye:
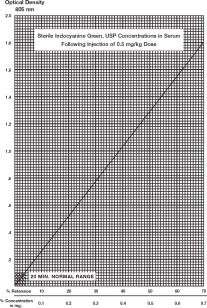
A typical curve obtained by plotting dye concentration versus optical density is shown. The percent retention can be read from this plot. If more accurate results are desired, a curve using the patient's blood and the vial of Indocyanine Green for injection being used in the determination can be constructed as follows:
- 1.
- Take 6 mL of non-dye-containing venous blood from the patient's arm. Place in a test tube and allow the blood to clot. The serum should be separated by centrifugation.
- 2.
- Pipette 1 mL of the serum into a microcuvette.
- 3.
- Add 1 lambda (λ) of the 5 mg/mL aqueous Indocyanine Green for injection (sterile indocyanine green) solution to the serum, giving a dilution of 5 mg/liter, the standard for 50% retention. (The addition of 2 lambda (λ) of the 5 mg/mL Indocyanine Green for injection solution would give 100% retention; however, this concentration cannot be read on the spectrophotometer.)
- 4.
- The optical density of this solution should be read at 805 nm, using normal serum as the blank.
- 5.
- Using graph paper similar to that used in the illustration, plot the 50% figure obtained in Step 4, and draw a line connecting this point with the zero coordinates.
Percentage Retention: A single 20-minute sample (withdrawn from a vein in the opposite arm to that injected) should be collected and allowed to clot, centrifuged and its optical density determined at 805 nm using the patient's normal serum as the blank. The dye concentration can be read from the curve above. A single 20-minute sample of serum in healthy subjects should contain no more than 4% of the initial concentration of the dye. The use of percentage retention is less accurate than percentage disappearance rate. Hemolysis is not expected to interfere with a reading.
Determination Using Disappearance Rate of Dye: To calculate the percentage disappearance rate, obtain samples at 5, 10, 15 and 20 minutes after injecting the dye. Prepare the sample as in the previous section and measure the optical densities at 805 nm, using the patient's normal serum as the blank. The Indocyanine Green for injection concentration in each timed specimen should be determined by using the concentration curve illustrated. Values should be plotted on semilogarithmic paper.
Specimens containing Indocyanine Green for injection should be read at the same temperature since its optical density is influenced by temperature variations.
Normal Values: Percentage disappearance rate in healthy subjects is 18 to 24% per minute. Normal biological half-time is 2.5 to 3.0 minutes.
2.3 Ophthalmic Angiography Studies
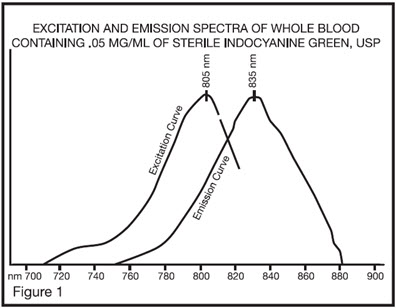
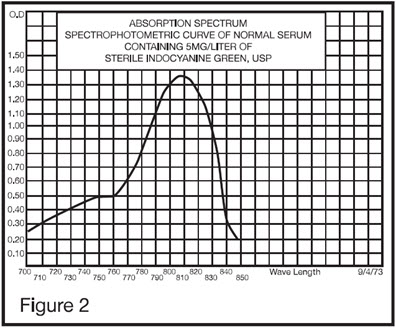
The excitation and emission spectra (Figure 1) and the absorption spectra (Figure 2) of Indocyanine Green for injection make it useful in ophthalmic angiography.
Dosages up to 40 mg Indocyanine Green for injection dye in 2 mL of Sterile Water for injection should be used, depending on the imaging equipment and technique used. The antecubital vein can be injected with an Indocyanine Green for injection dye bolus and should immediately be followed by a 5 mL bolus of normal saline.
3 DOSAGE FORMS AND STRENGTHS
Indocyanine Green for injection USP is a sterile, lyophilized green powder containing 25 mg of indocyanine green with no more than 5% sodium iodide.
4 CONTRAINDICATIONS
Indocyanine Green for injection contains sodium iodide and should be used with caution in patients who have a history of allergy to iodides because of the risk of anaphylaxis.
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis
Deaths from anaphylaxis have been reported following Indocyanine Green for injection administration during cardiac catheterization.
5.2 Drug Instability
Indocyanine Green for injection is unstable in aqueous solution and must be used within 6 hours. However, the dye is stable in plasma and whole blood so that samples obtained in discontinuous sampling techniques may be read hours later. Sterile techniques should be used in handling the dye solution as well as in the performance of the dilution curves. If a precipitate is present, discard the solution.
6 ADVERSE REACTIONS
Anaphylactic or urticarial reactions have been reported in patients with or without history of allergy to iodides. If such reactions occur, treat with the appropriate agents, e.g., epinephrine, antihistamines, and corticosteroids.
7 DRUG INTERACTIONS
Preparations containing sodium bisulfite, including some heparin products reduce the absorption peak of Indocyanine Green for injection in blood and, therefore, should not be used as an anticoagulant for the collection of samples for analysis.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted with Indocyanine Green for injection. It is also not known whether Indocyanine Green for injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Indocyanine Green for injection should be given to a pregnant woman only if clearly indicated.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Indocyanine Green for injection is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have been established. See DOSAGE AND ADMINISTRATION (2) for specific dosing information in pediatric patients.
10 OVERDOSAGE
There are no data available describing the signs, symptoms, or laboratory findings accompanying overdosage. The LD50 after intravenous administration ranges between 60 and 80 mg/kg in mice, 50 and 70 mg/kg in rats and 50 and 80 mg/kg in rabbits. Based on body surface area, these doses are 2.4 to 13-fold the maximum recommended human (MRHD) dose of 2 mg/kg for indicator-dilution studies, 10 to 52-fold the MRHD of 0.5 mg/kg for hepatic-function studies, and 7 to 39‑fold the MRHD of 0.67 mg/kg for ophthalmic angiography studies.
11 DESCRIPTION
Indocyanine Green for injection USP is a sterile, lyophilized green powder containing 25 mg of indocyanine green with no more than 5% sodium iodide. It is packaged with Sterile Water for injection, USP used to dissolve the indocyanine green. Indocyanine Green for injection USP is to be administered intravenously.
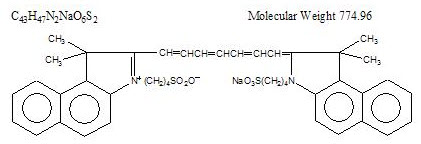
Indocyanine green is a water soluble, tricarbocyanine dye with a peak spectral absorption at 800 nm. The chemical name for Indocyanine Green is 1 H-Benz[e]indolium, 2-[7-[1,3-dihydro-1,1-dimethyl-3-(4-sulfobutyl)-2H-benz[e] indol-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-,hydroxide, inner salt, sodium salt. Indocyanine Green for injection USP has a pH of approximately 6.5 when reconstituted. Each vial of Indocyanine Green for injection USP contains 25 mg of indocyanine green as a sterile lyophilized powder.
12 CLINICAL PHARMACOLOGY
Indocyanine Green for injection permits recording of the indicator-dilution curves for both diagnostic and research purposes independently of fluctuations in oxygen saturation. Following intravenous injection, Indocyanine Green for injection is rapidly bound to plasma protein, of which albumin is the principle carrier (95%). Indocyanine Green for injection undergoes no significant extrahepatic or enterohepatic circulation; simultaneous arterial and venous blood estimations have shown negligible renal, peripheral, lung or cerebro-spinal uptake of the dye. Indocyanine Green for injection is taken up from the plasma almost exclusively by the hepatic parenchymal cells and is secreted entirely into the bile. After biliary obstruction, the dye appears in the hepatic lymph, independently of the bile, suggesting that the biliary mucosa is sufficiently intact to prevent diffusion of the dye, though allowing diffusion of bilirubin. These characteristics make Indocyanine Green for injection a helpful index of hepatic function.
The peak absorption and emission of Indocyanine Green for injection lie in a region (800 to 850 nm) where transmission of energy by the pigment epithelium is more efficient than in the region of visible light energy. Indocyanine Green for injection also has the property of being nearly 98% bound to blood protein, and therefore, excessive dye extravasation does not take place in the highly fenestrated choroidal vasculature. It is, therefore, useful in both absorption and fluorescence infrared angiography of the choroidal vasculature when using appropriate filters and film in a fundus camera.
The plasma fractional disappearance rate at the recommended 0.5 mg/kg dose has been reported to be significantly greater in women than in men, although there was no significant difference in the calculated value for clearance.
16 HOW SUPPLIED/STORAGE AND HANDLING
Indocyanine Green for injection USP is supplied in a kit (NDC 70100-424-02) containing six 25 mg Indocyanine Green for injection USP vials and six 10 mL Sterile Water for injection, USP plastic vials:
NDC 70100-424-01 Indocyanine Green for injection USP vial. 25 mg fill in 25 mL vial.
NDC 63323-185-10 (or NDC 0409-4887-17) Sterile Water for injection, USP, 10 mL fill in 10 mL plastic vials.
Each combination of Indocyanine Green for injection USP vial and Sterile Water for injection, USP plastic vial is intended for single-patient-use.
STORAGE: Store at 20° to 25°C (68° to 77°F).
Manufactured by:
Patheon Italia S.p.A.
20900 Monza (MB), ITALY
Distributed by:
Diagnostic Green LLC
Farmington Hills, MI 48331 USA
Sterile Water for injection,
USP is manufactured by:
Fresenius Kabi USA, LLC
Grand Island, NY 14072 USA
or
Hospira, Inc.
Rocky Mount, NC 27804 USA
50426
PRINCIPAL DISPLAY PANEL - Vial
NDC 70100-424-01
Indocyanine Green
for Injection, USP
25 mg/Vial
Single-Patient-Use
After reconstitution, use within 6 hours.
Rx only Sterile
Distributed by: Diagnostic Green LLC
50428
04/2022
Lot No.
Exp.:
PRINCIPAL DISPLAY PANEL - STERILE WATER VIAL
10 mL Single-dose
Sterile Water
for Injection, USP
FOR DRUG DILUENT USE
Rx only NDC 0409-4887-17
Contains no antimicrobial or other added
substance. Sterile, nonpyrogenic. Do not give
intravenously unless rendered nearly isotonic.
Hospira, Inc. RL-4428
Lake Forest, IL 60045 USA
PRINCIPAL DISPLAY PANEL - Carton
NDC 70100-424-02
Indocyanine Green
for Injection, USP
For Intravenous Administration 25 mg/Vial Kit Rx Only - Sterile
Distributed by: Diagnostic Green LLC
Back Panel
NDC 70100-424-02
DIRECTIONS FOR USE ENCLOSED
CAUTION: To ensure accurate readings,
Indocyanine Green, USP dissolved in Sterile Water
for Injection, USP must be used within 6 hours.
STORAGE: Store at 20° to 25°C (68° to 77°F)
[See USP Controlled Room Temperature].
USAGE: See package insert for dosage information.
Contents: Six Indocyanine Green for Injection,
USP vials (25 mg each)
Six Sterile Water for Injection, USP Vials
(10 mL each)
Indocyanine Green
for Injection, USP
For Intravenous Administration 25 mg/Vial Kit Rx Only - Sterile
Right Panel
Indocyanine Green for
Injection, USP
Distributed by:
Diagnostic Green LLC
Farmington Hills MI 48331 USA
Manufactured by:
Patheon Italia S.p.A.
20052 Monza (Milano) ITALY
Sterile Water Manufactured by:
Hospira, Inc.
Rocky Mount, NC 27804
or
Fresenius Kabi USA, LLC
Grand Island, NY 14072
06/2018
50427