FULL PRESCRIBING INFORMATION
1. INDICATIONS AND USAGE
Tonsillitis and/or Pharyngitis
MOXATAG is a penicillin-class antibacterial indicated for the treatment of tonsillitis and/or pharyngitis secondary to Streptococcus pyogenes (S. pyogenes) in adults and pediatric patients 12 yrs and older.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of MOXATAG and other antibacterial drugs, MOXATAG should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2. DOSAGE AND ADMINISTRATION
Tonsillitis and/or Pharyngitis
The recommended dose of MOXATAG is 775 mg once daily taken within 1 hour of finishing a meal for 10 days. The full 10-day course of therapy should be completed for effective treatment of tonsillitis and/or pharyngitis secondary to S. pyogenes.
Do not chew or crush tablet.
3. DOSAGE FORMS AND STRENGTHS
775 mg blue film-coated, oval-shaped tablets printed with ”MB-111” on one side in black edible ink.
4. CONTRAINDICATIONS
MOXATAG is contraindicated in patients with known serious hypersensitivity to amoxicillin or to other drugs in the same class or patients who have demonstrated anaphylactic reactions to beta-lactams.
5. WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis and Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy. Although anaphylaxis is more frequent following parenteral therapy, it has occurred in patients on oral penicillins. These reactions are more likely to occur in individuals with a history of penicillin hypersensitivity and/or a history of sensitivity to multiple allergens. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe reactions when treated with cephalosporins. Before initiating therapy with MOXATAG, careful inquiry should be made concerning previous hypersensitivity reactions to penicillins, cephalosporins, or other allergens. If an allergic reaction occurs, MOXATAG should be discontinued and appropriate therapy instituted.
Serious anaphylactic reactions require immediate emergency treatment with epinephrine. Oxygen, intravenous steroids, and airway management, including intubation, should also be administered as indicated.
5.2 Clostridium difficile Associated Diarrhea (CDAD)
Clostridium difficile Associated Diarrhea (CDAD) has been reported with nearly all antibacterial agents, including amoxicillin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile. C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.3 Superinfections
The possibility of superinfections with mycotic or bacterial pathogens should be kept in mind during therapy. If superinfections occur, amoxicillin should be discontinued and appropriate therapy instituted.
5.4 Mononucleosis Rash
A high percentage of patients with mononucleosis who receive ampicillin develop an erythematous skin rash. Thus, ampicillin-class antibiotics should not be administered to patients with mononucleosis.
5.5 Development of Drug-Resistant Bacteria
Prescribing amoxicillin in the absence of proven or strongly suspected bacterial infection or treating prophylactically is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.6 False-Positive Urinary Glucose Tests
High urine concentrations of ampicillin may result in false-positive reactions when testing for the presence of glucose in urine using Clinitest®, Benedict's Solution or Fehling's Solution. Since this effect may also occur with amoxicillin, it is recommended that glucose tests based on enzymatic glucose oxidase reactions (such as Clinistix®) be used.
6. ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Study Experience
Tonsillitis and/or Pharyngitis
In a controlled Phase 3 trial, 302 adult and pediatric patients (≥ 12 years) were treated with MOXATAG 775 mg once-daily for 10 days and 306 adult and pediatric patients (≥ 12 years) were treated with penicillin VK 250 mg QID for 10 days.
In this clinical trial, the majority of treatment-emergent adverse reactions were of a mild and transient nature with similar frequency reported in both treatment groups. Discontinuation due to drug-related treatment-emergent adverse reactions occurred in 1.3 % of the MOXATAG-treated patients and 3.3 % of the penicillin VK-treated patients.
The most frequently reported adverse reactions (≥ 1%) which were suspected or probably drug-related are shown in Table 1.
|
*Presented in decreasing order of frequency in the MOXATAG column within each system organ class. |
||
| Number (%) of patients | ||
| System Organ Class/Preferred Term* | MOXATAG (N =302) | Pen VK (N = 306 ) |
| Patients with at least one drug-related treatment-emergent adverse event | 32 (10.6) | 45 (14.7) |
| Infections and infestations | ||
| Vulvovaginal mycotic infection | 6 (2.0) | 8 (2.6) |
| Gastrointestinal disorders | ||
| Diarrhea | 5 (1.7) | 6 (2.0) |
| Nausea | 4 (1.3) | 2 (0.7) |
| Vomiting | 2 (0.7) | 5 (1.6) |
| Abdominal pain | 1 (0.3) | 3 (1.0) |
| Nervous system disorders | ||
| Headache | 3 (1.0) | 3 (1.0) |
6.2 Adverse Reactions for Other Amoxicillin Products
The following adverse reactions have been reported for other products containing amoxicillin:
Infections and Infestations: Mucocutaneous candidiasis.
Gastrointestinal: Nausea, vomiting, diarrhea, and hemorrhagic/ pseudomembranous colitis.
Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment.
Hypersensitivity Reactions: Anaphylaxis (See Warnings and Precautions)
Serum sickness like reactions, erythematous maculopapular rashes, erythema multiforme, Stevens-Johnson Syndrome, exfoliative dermatitis, toxic epidermal necrolysis, acute generalized exanthematous pustulosis, hypersensitivity vasculitis and urticaria have been reported. (NOTE: These hypersensitivity reactions may be controlled with antihistamines and, if necessary, systemic corticosteroids. Whenever such reactions occur, amoxicillin should be discontinued unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to amoxicillin therapy.)
Liver: A moderate rise in AST (SGOT) and/or ALT (SGPT) has been noted, but the significance of this finding is unknown. Hepatic dysfunction including cholestatic jaundice, hepatic cholestasis and acute cytolytic hepatitis have been reported.
Hemic and Lymphatic Systems: Anemia, including hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia, and agranulocytosis have been reported during therapy with penicillins. These reactions are usually reversible on discontinuation of therapy and are believed to be hypersensitivity phenomena.
Central Nervous System: Reversible hyperactivity, agitation, anxiety, insomnia, confusion, convulsions, behavioral changes, and/or dizziness have been reported rarely.
Renal: Crystalluria has also been reported
Miscellaneous: Tooth discoloration (brown, yellow, or gray staining) has been rarely reported. Most reports occurred in pediatric patients. Discoloration was reduced or eliminated with brushing or dental cleaning in most cases.
7. DRUG INTERACTIONS
7.1 Probenecid
Probenecid decreases the renal tubular secretion of amoxicillin. Concurrent use of MOXATAG and probenecid may result in increased and prolonged blood levels of amoxicillin. The clinical relevance of this finding has not been evaluated.
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects. Pregnancy Category B. Reproduction studies have been performed in mice and rats at doses up to 2000 mg/kg (12.5 and 25 times the human dose in mg/m2) and have revealed no evidence of impaired fertility or harm to the fetus due to amoxicillin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.2 Labor and Delivery
Oral ampicillin-class antibiotics are poorly absorbed during labor. Studies in guinea pigs showed that intravenous administration of ampicillin slightly decreased the uterine tone and frequency of contractions but moderately increased the height and duration of contractions. However, it is not known whether use of amoxicillin in humans during labor or delivery has immediate or delayed adverse effects on the fetus, prolongs the duration of labor, or increases the likelihood that forceps delivery or other obstetrical intervention or resuscitation of the newborn will be necessary.
8.3 Nursing Mothers
Penicillins have been shown to be excreted in human milk. Amoxicillin use by nursing mothers may lead to sensitization of infants. Caution should be exercised when amoxicillin is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of MOXATAG in pediatric patients 12 years of age and older have been established based on results of a clinical trial that included adults and pediatric patients (12 years or older). [see Clinical Studies (14)] Sixty three (21 %) of the study participants were pediatric patients 12 years of age or older. There were no significant differences in treatment response or adverse reactions from adults.
The safety and effectiveness of MOXATAG in pediatric patients younger than 12 years has not been established.
8.5 Geriatric Use
Clinical studies with MOXATAG did not include a sufficient number of patients aged 65 years and over to determine whether they respond differently from younger patients. Other reported clinical experiences with amoxicillin have not yet identified differences in responses between the elderly and younger patients, but a greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Renal Impairment
MOXATAG has not been studied in patients with renal impairment; however, a reduction of amoxicillin dose is generally recommended for patients with severe renal impairment. Therefore, MOXATAG is not recommended for use in patients with severe renal impairment (CrCl <30 mL/min) or patients on hemodialysis.
10. OVERDOSAGE
In case of overdose, discontinue medication, treat symptomatically, and institute supportive measures as required. If the overdose is very recent and there is no contraindication, an attempt at emesis or other means of removal of drug from the stomach may be performed. A prospective study of 51 pediatric patients at a poison-control center suggested that overdosages of less than 250 mg/kg of amoxicillin are not associated with significant clinical symptoms and do not require gastric emptying.
Interstitial nephritis resulting in oliguric renal failure has been reported in a small number of patients after overdosage with amoxicillin.
Crystalluria, in some cases leading to renal failure, has also been reported after amoxicillin overdosage in adult and pediatric patients. In case of overdosage, adequate fluid intake and diuresis should be maintained to reduce the risk of amoxicillin crystalluria.
Renal impairment appears to be reversible with cessation of drug administration. High blood levels may occur more readily in patients with impaired renal function because of decreased renal clearance of amoxicillin. Amoxicillin may be removed from circulation by hemodialysis.
For additional information about overdose treatment, call a poison control center (1-800-222-1222).
11. DESCRIPTION
MOXATAG (amoxicillin extended-release) tablets for oral administration are provided as blue film-coated tablets that contain 775 mg of amoxicillin as the trihydrate and are printed with ”MB-111” on one side in black edible ink.
Amoxicillin is a semi-synthetic antibiotic, an analog of ampicillin, with bactericidal activity against gram-positive and gram-negative microorganisms.
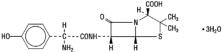
Chemically, amoxicillin is (2 S,5 R,6 R)-6-[(R)-(-)-2-amino-2-(p-hydroxyphenyl) acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate. Its chemical name is amoxicillin. It may be represented structurally as:
The amoxicillin molecular formula is C16H19N3O5S·3H2O, and the molecular weight is 419.45.
MOXATAG is an extended release tablet formulation consisting of three components, one immediate-release and two delayed-release, each containing amoxicillin. The three components are combined in a specific ratio to prolong the release of amoxicillin from MOXATAG compared to immediate-release amoxicillin.
Each tablet contains amoxicillin, crospovidone, FD&C Blue #2 lake, hypromellose, hypromellose acetate succinate, iron oxide, magnesium stearate, methacrylic acid copolymer, microcrystalline cellulose, polyethylene glycol 400, polyoxyl 35 castor oil, shellac, colloidal silicon dioxide, sodium lauryl sulfate, talc, titanium dioxide, and triethyl citrate.
12. CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
MOXATAG is an extended-release formulation of amoxicillin intended to provide once-daily dosing. Following the administration of MOXATAG with a low-fat meal in healthy subjects, mean amoxicillin AUC0-∞, Cmax, and Tmax values were 29.8 μg•h/mL, 6.6 μg/mL and 3.1 hours, respectively. The mean plasma concentration-time curve is shown below in Figure 1.
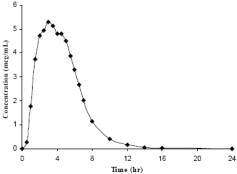
Figure 1. Mean Amoxicillin Plasma Concentrations Following a Single Oral Dose of MOXATAG With a Low-Fat Meal in Healthy Subjects (N=20)
Administration of MOXATAG with food decreases the rate, but not the extent of amoxicillin absorption. Compared to immediate-release amoxicillin suspension, the rate of amoxicillin absorption following administration of MOXATAG was slower, resulting in a lower Cmax and longer Tmax. Total amoxicillin exposure (AUC) achieved with MOXATAG is similar to that observed after oral administration of a comparable dose of immediate-release amoxicillin suspension.
Amoxicillin diffuses readily into most body tissues and fluids, with the exception of brain and spinal fluid, except when meninges are inflamed. Amoxicillin is approximately 20% protein bound in human serum.
Amoxicillin is primarily cleared by renal excretion. Approximately 60% of an oral dose of immediate-release amoxicillin is eliminated unchanged in urine. The half-life of amoxicillin after oral administration of MOXATAG is approximately 1.5 hours, similar to that of immediate-release amoxicillin. No accumulation of amoxicillin was observed after once-daily dosing of 775 mg of MOXATAG for 7 days.
Drug Interactions
In a study of healthy adult subjects, amoxicillin AUC was similar whereas Cmax increased approximately 35% following the administration of lansoprazole with MOXATAG given with food.
Probenecid decreases the renal tubular secretion of amoxicillin. Concurrent use of MOXATAG and probenecid may result in increased and prolonged blood levels of amoxicillin. The clinical relevance of this finding has not been evaluated.
12.4 Microbiology
Amoxicillin is a semi-synthetic antimicrobial belonging to the penicillin class of antimicrobials with activity against gram-positive bacteria.
Mechanism of Action
Amoxicillin exerts its bactericidal action against susceptible organisms during the stage of multiplication. It acts through the inhibition of biosynthesis of cell wall mucopeptide.
Mechanism of Resistance
To date there are no known mechanisms of resistance to penicillin or amoxicillin in Streptococcus pyogenes.
MOXATAG has been shown to be active in vitro against isolates of the microorganism S. pyogenes and in clinical infections as described in the INDICATIONS AND USAGE section.
Streptococcus pyogenes
The following in vitro data are available, but their clinical significance is unknown. At least 90% of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptibility breakpoint of amoxicillin (as determined by susceptibility tests using the class representative agents penicillin or ampicillin).
Susceptibility Test Methods:
When available, the clinical microbiology laboratory should provide cumulative results of the in vitro susceptibility test results for antimicrobial drugs used in local hospitals and practice areas to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting the most effective antimicrobial.
Susceptibility testing of penicillins (such as amoxicillin) and other β-lactams approved by FDA for the treatment of Group A streptococcus (S. pyogenes) is not routinely necessary for clinical purposes. Isolates of Group A streptococcus resistant to amoxicillin have not been recognized and therefore all isolates can be considered susceptible to amoxicillin However, susceptibility tests can be conducted using dilution or diffusion techniques employing penicillin or ampicillin to predict susceptibility to amoxicillin.
Dilution Techniques
Quantitative methods are used to determine antimicrobial MICs. These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure1,3. Standardized procedures are based on a dilution method (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of penicillin or ampicillin. The susceptibility of Group A streptococcus to penicillin or amoxicillin should be interpreted according to the criteria in Table 2.
Diffusion Technique
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of a standardized inoculum concentration. This procedure uses paper disks impregnated with 10 units penicillin or 10 mcg ampicillin to test the susceptibility of S. pyogenes to penicillin or amoxicillin. Reports from the laboratory providing results of the standard single-disk susceptibility test should be interpreted according to the criteria in Table 2
| Antimicrobial | Minimum Inhibitory Concentration (mcg/mL) | Disk Diffusion (zone diameter in mm) |
| Penicillin | ≤0.12 = Susceptible | ≥24 = Susceptible |
| Ampicillin | ≤0.25 = Susceptible | ≥24 = Susceptible |
The current absence of data on resistant isolates precludes defining any categories other than “Susceptible”. Isolates yielding results suggestive of a “nonsusceptible” category should be retested, and if the result is confirmed, the isolate should be submitted to a reference laboratory for further testing.
Quality Control
Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of the supplies and reagents used in the assay, and the techniques of the individuals performing the test. Standard penicillin or ampicillin powders should provide the MIC ranges provided in Table 3. For the disk diffusion technique using the 10 unit penicillin disk or 10 μg ampicillin disk the criteria in Table 3 should be achieved.
|
ATCC = American Type Culture Collection |
|||
| QC Organism | Antimicrobial | Minimum Inhibitory Concentration (mcg/mL) | Disk Diffusion (zone diameter in mm) |
| Streptococcus pneumoniae | Penicillin | 0.25 – 1 | 24 – 30 |
| ATCC 49619 | Ampicillin | 0.06 – 0.25 | 30 - 36 |
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. Studies to detect mutagenic potential of amoxicillin alone have not been conducted; however, the following information is available from tests on a 4:1 mixture of amoxicillin and potassium clavulanate (Augmentin). Augmentin was non-mutagenic in the Ames bacterial mutation assay, and the yeast gene conversion assay. Augmentin was weakly positive in the mouse lymphoma assay, but the trend toward increased mutation frequencies in this assay occurred at doses that were also associated with decreased cell survival. Augmentin was negative in the mouse micronucleus test, and in the dominant lethal assay in mice. Potassium clavulanate alone was tested in the Ames bacterial mutation assay and in the mouse micronucleus test, and was negative in each of these assays. In a multi-generation reproduction study in rats, no impairment of fertility or other adverse reproductive effects were seen at doses up to 500 mg/kg (approximately 6 times the human dose in mg/m2).
14. CLINICAL STUDIES
In a randomized, parallel-group, multi-center, double-blind, double-dummy study in adults and pediatrics (age≥ 12 years) with tonsillitis and/or pharyngitis secondary to S. pyogenes, MOXATAG 775 mg QD for 10 days was non-inferior to penicillin VK 250 mg QID for 10 days.
Using strict evaluability and microbiologic response criteria 4-8 days post-therapy, the following bacteriological eradication rates and statistical outcomes in the per-protocol (PPb) and modified intent-to-treat (mITT) populations were obtained (Table 4). The mITT population included all randomized patients with a positive throat culture for S. pyogenes at baseline. The PPb population included mITT patients who had post-therapy cultures, were compliant with treatment, and didn't have major protocol violations.
| Study Population | MOXATAG | Penicillin VK | Rate Difference 95% CI (%) |
| PPb | 198/233 (85.0%) | 191/229 (83.4%) | 1.6 (-5.1, 8.2) |
| mITT | 204/256 (79.7%) | 206/264 (78.0%) | 1.7 (-5.4, 8.7) |
15. REFERENCES
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—7th ed Clinical and Laboratory Standards Institute document M7-A7. Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2006.
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—9th ed. CLSI document M2-A9 CLSI, Wayne, PA 19087-1898 2006.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; 17th Informational Supplement. CLSI document M100-S17 CLSI, Wayne, PA 19087-1898, 2007.
16. HOW SUPPLIED/STORAGE AND HANDLING
MOXATAG tablets for oral administration are provided as blue film-coated, oval-shaped tablets that contain 775 mg of amoxicillin as the trihydrate. The tablets are printed with “MB-111” on one side in black edible ink. MOXATAG is packaged in bottles and blister cards as follows:
- Bottles of 30 NDC 11042-142-03
- 10 Tablet Card
(1 tablet per blister cavity) NDC 11042-142-02
17. PATIENT COUNSELING INFORMATION
17.1 Instructions for Administration of MOXATAG
Patients should be informed that the recommended dose of MOXATAG is 775 mg once daily taken with food for 10 days. MOXATAG should be taken within 1 hour of finishing a meal and at approximately the same time every day.The full 10 day course of therapy should be completed for effective treatment of tonsillitis and/or pharyngitis secondary to S. pyogenes. Patients should be instructed not to chew or crush the tablet. No other forms of immediate-release amoxicillin can be substituted for MOXATAG.
17.2 Hypersensitivity Reactions
Patients should be informed that serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported while on penicillin therapy. Patients should be questioned regarding any hypersensitivity reactions to penicillins, cephalosporins or other allergens. Whenever such reactions occur, the patient should be instructed to contact their physician immediately. Serious anaphylactic reactions require immediate emergency treatment with epinephrine. Oxygen, intravenous steroids, and airway management, including intubation, should also be administered as indicated.
17.3 Clostridium difficile Associated Diarrhea
Patients should be informed that diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
AUGMENTIN is a registered trademark of GlaxoSmithKline.
CLINITEST is a registered trademark of Miles, Inc.
CLINISTIX is a registered trademark of Bayer Corporation.
MOXATAG is a trademark of MiddleBrook Pharmaceuticals, Inc., all rights reserved.
Manufactured in Clonmel, Ireland by Stada Production Ireland
for
MiddleBrook Pharmaceuticals, Inc., Germantown, Maryland 20876
U.S. Patents 6,544,555; 6,669,948; 6,723,341
Issue Date 06/08
PC2049L/1
Copyright © 2008, MiddleBrook Pharmaceuticals, Inc. All rights reserved.