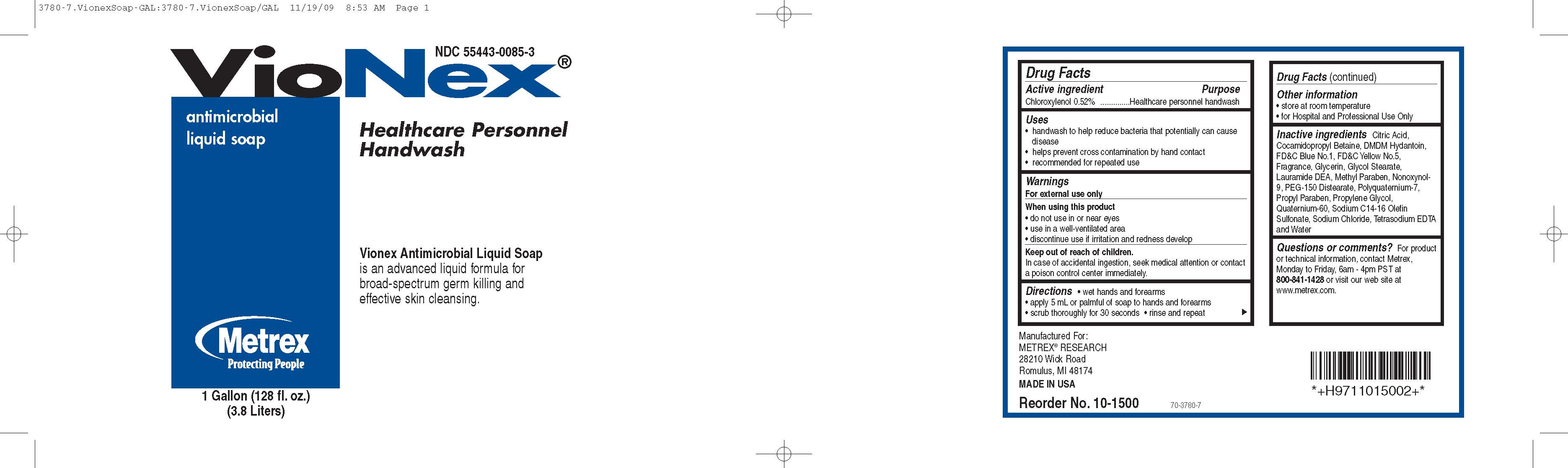
Drug Facts
 Active Ingredients Purpose
Active Ingredients Purpose
Chloroxylenol 0.52%..............................Healthcare personnel handwash
Uses
- handwash to help reduce bacteria that potentially can cause disease
- helps prevent cross contamination by hand contact
- recommended for repeated use
When using this product
- do not use in or near eyes
- use in a well-ventilated area
- discontinue use if irritation and redness develop
Keep out of reach of children
In case of accidental ingestion, seek medical attention or contact a poison control center immediately.
Directions
- wet hands and forearms
- apply 5 mL or palmful of soap to hands and forearms
- scrub thoroughly for 30 seconds
- rinse and repeat
Inactive Ingredients
Citric Acid, Cocamidopropyl Betaine, DMDM Hydantoin, F DC Blue No.1, FD C Yellow No.5, Fragrance, Glycerin, Glycol Stearate, Lauramide DEA, Methyl Paraben, Nonoxynol-9, PEG- 150 Distearate, Polyquaternium-7, Propyl Paraben, Propylene Glycol, Quaternium-60, Sodium C14-16 Olefin Sulfonate, Sodium Chloride, Tetrasodium EDTA and Water
Questions or comments?
For product or technical informaiton, contact Metrex, Monday to Friday, 6am - 4pm PST at 800-841-1428 or visit our website at www.metrex.com.
Principal Display Panel
 VioNex antimicrobial liquid soap
VioNex antimicrobial liquid soap
Healthcare Personnel Handwash
VioNex antimicrobial liquid soap is an advanced liquid formula for broad-spectrum germ killing and effective skin cleansing.
Manufactured for:
METREX RESEARCH
28210 Wick Road
Romulus, Michigan 48174
MADE IN USA
Reorder No.:
Metrex
Protecting People
fl. oz. ( mL)
gallon (fl. oz.)
( Liters)
For use in Wall Mount Dispenser (800 mL package only)