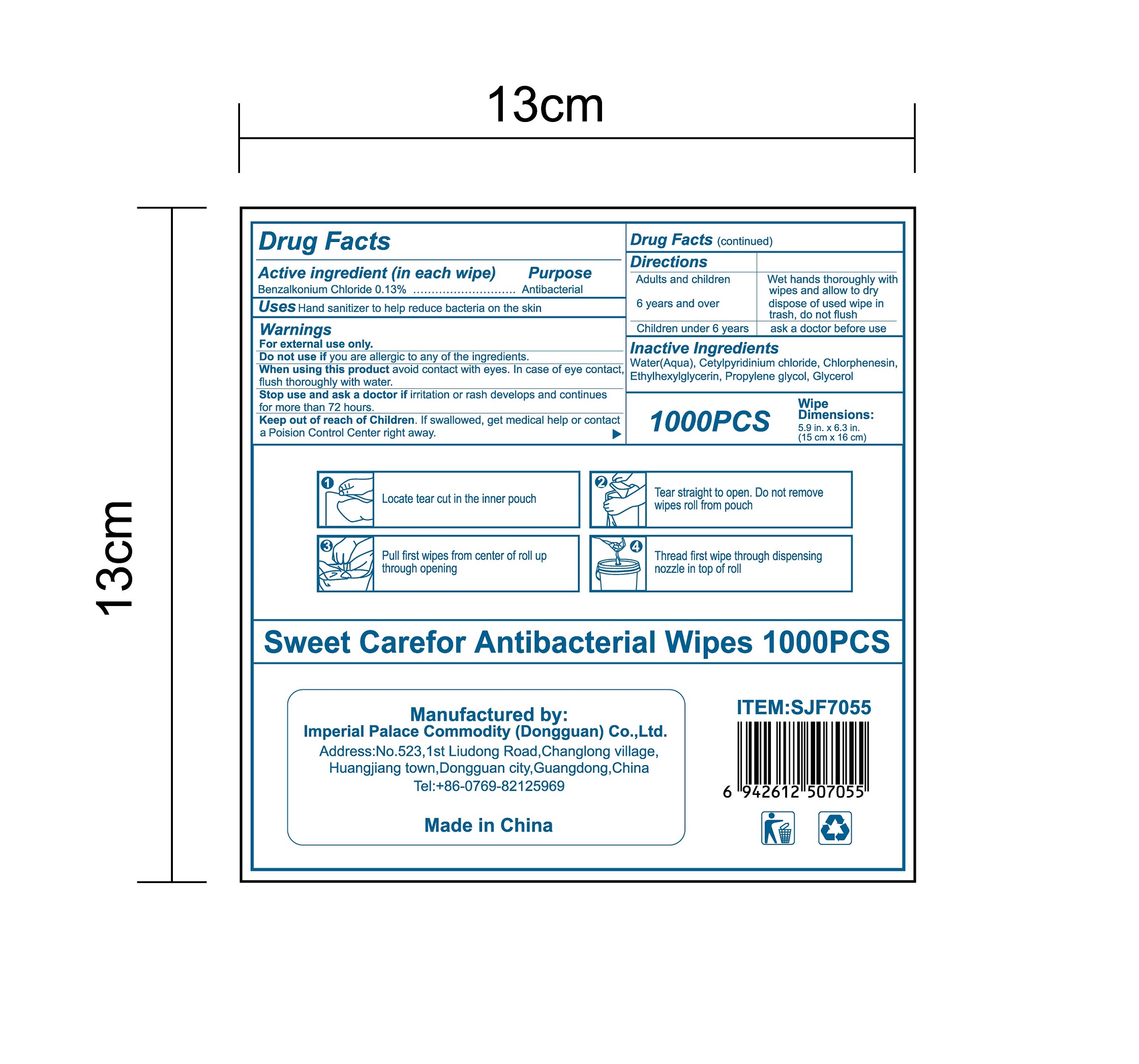
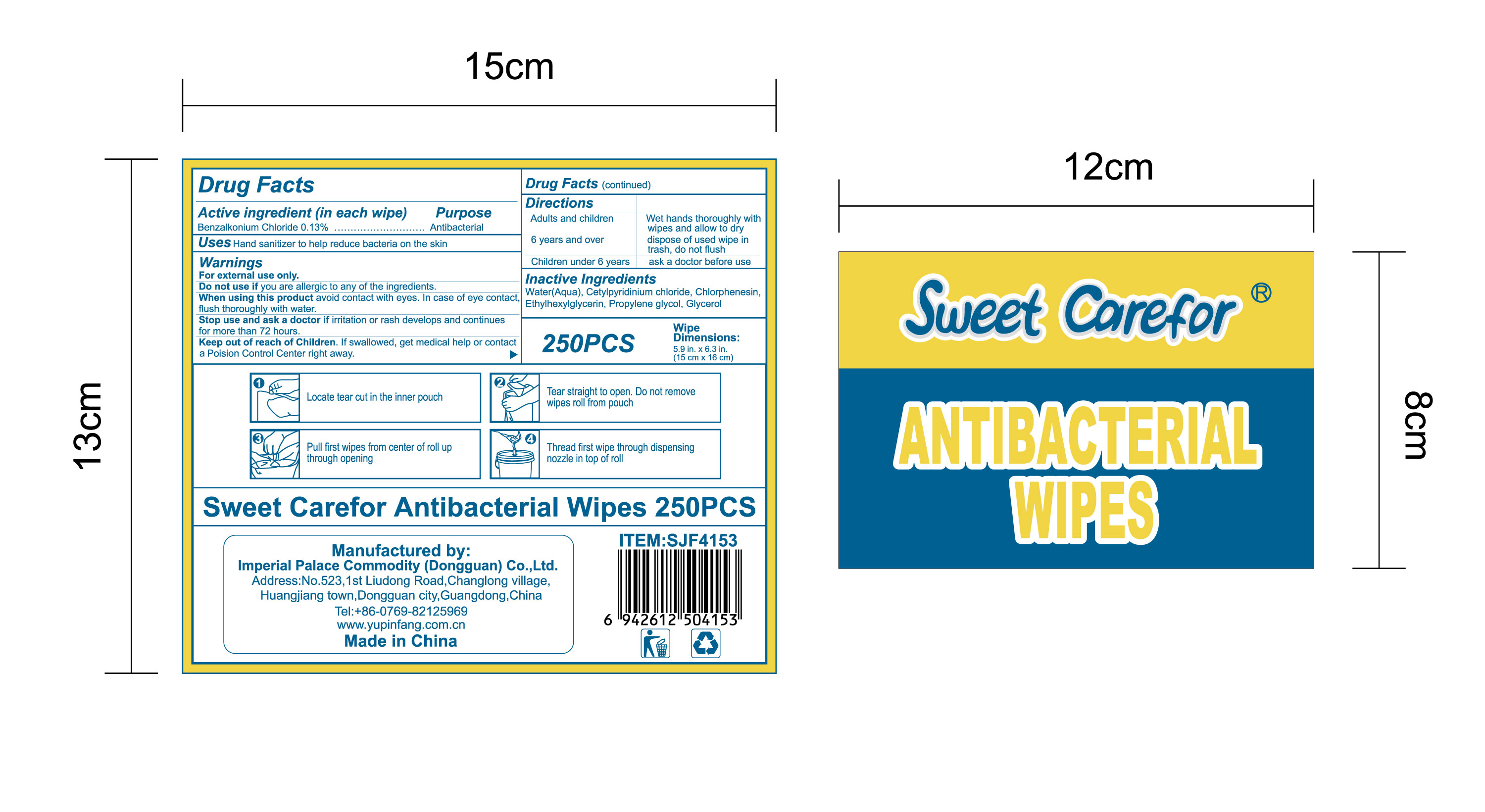
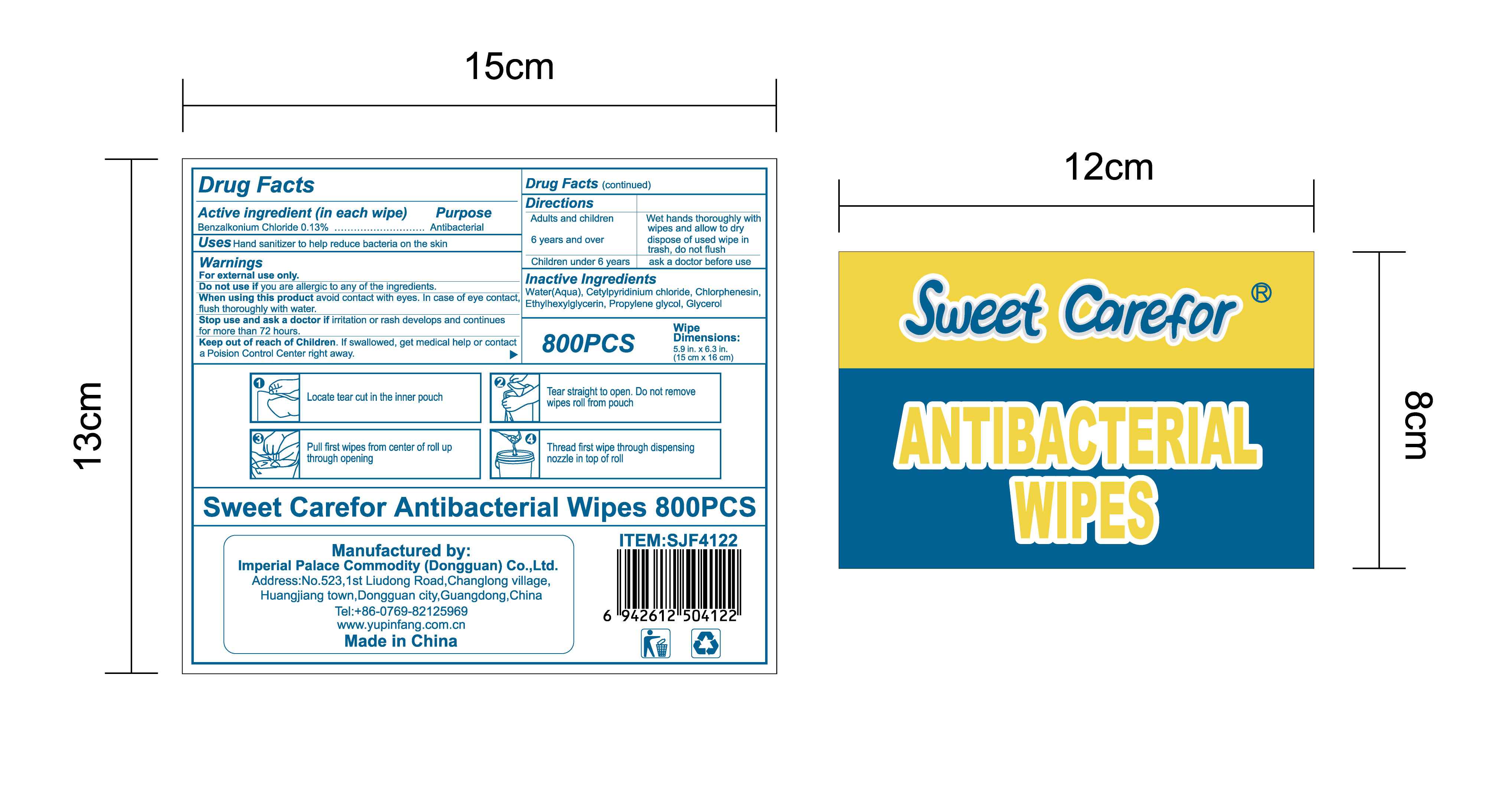
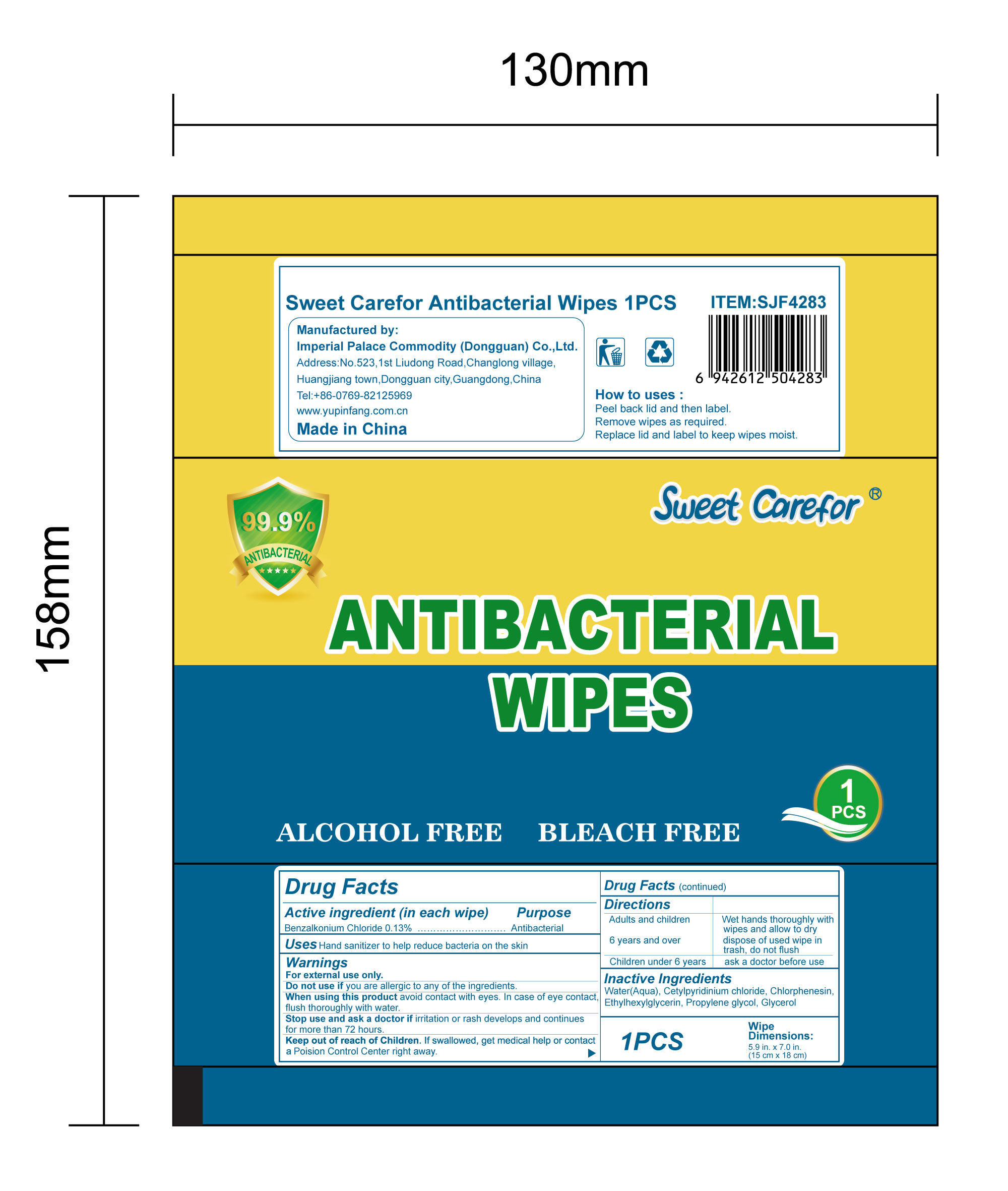
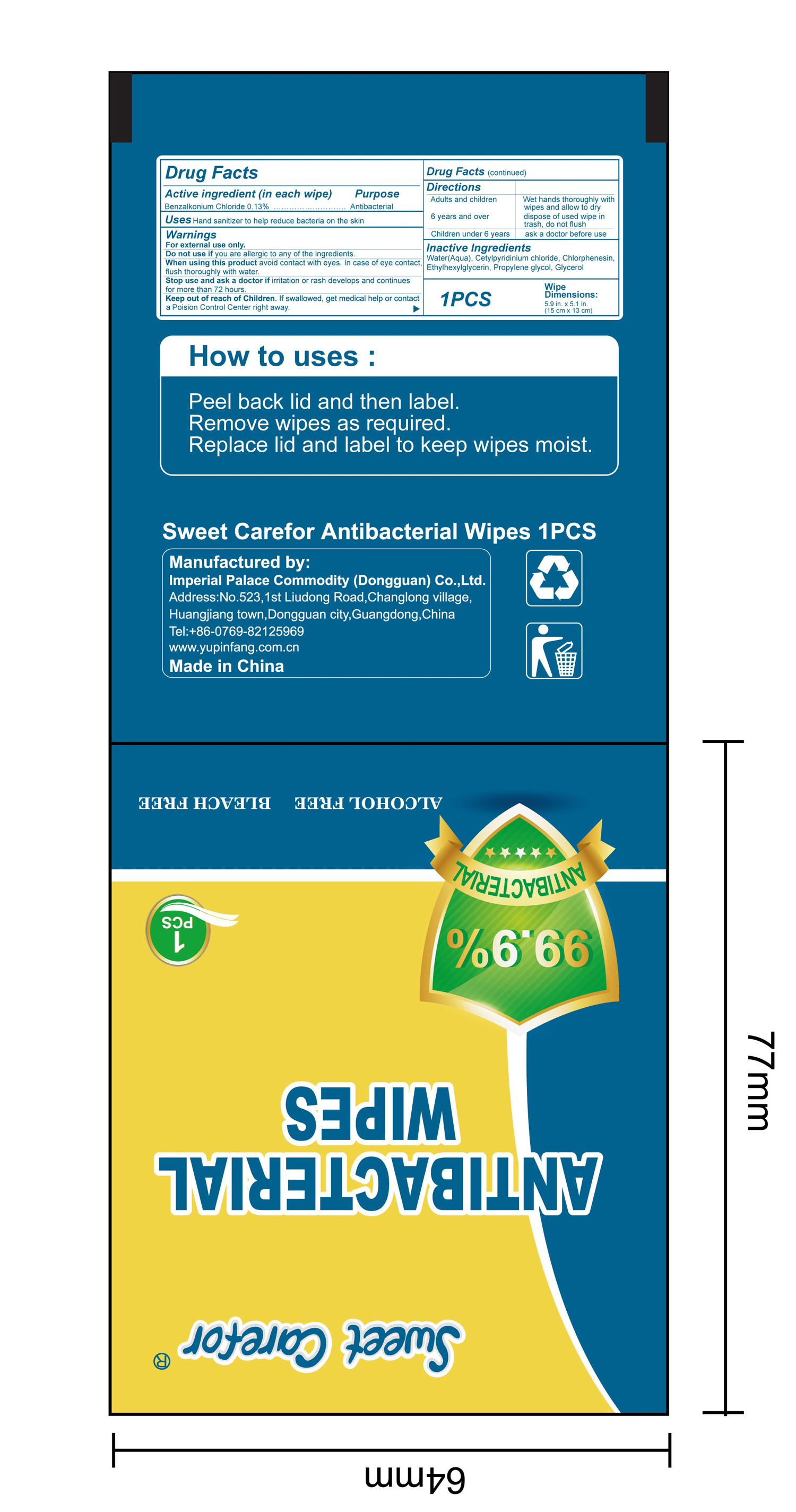
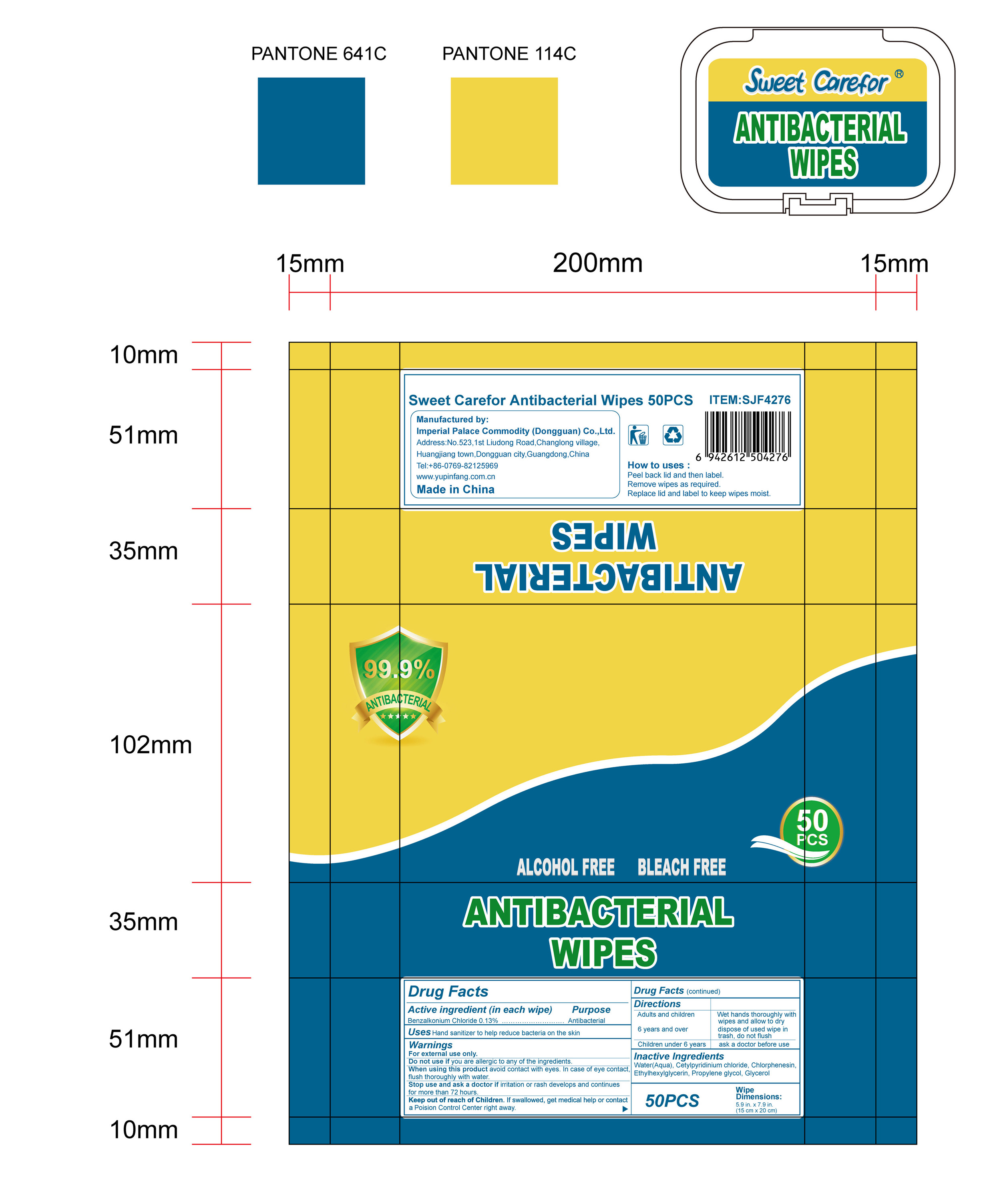
When using this product avoid contact with eyes. In case of eye contact,flush thoroughly with water.
Keep out of reach of Children. If swallowed, get medical help or contact a Poision Control Center right away.
Directions
Adults and children 6 years and over :
Wet hands thoroughly with wipes and allow to dry, dispose of used wipe in trash, don not flush
Children under 6 years:
ask a doctor before use.
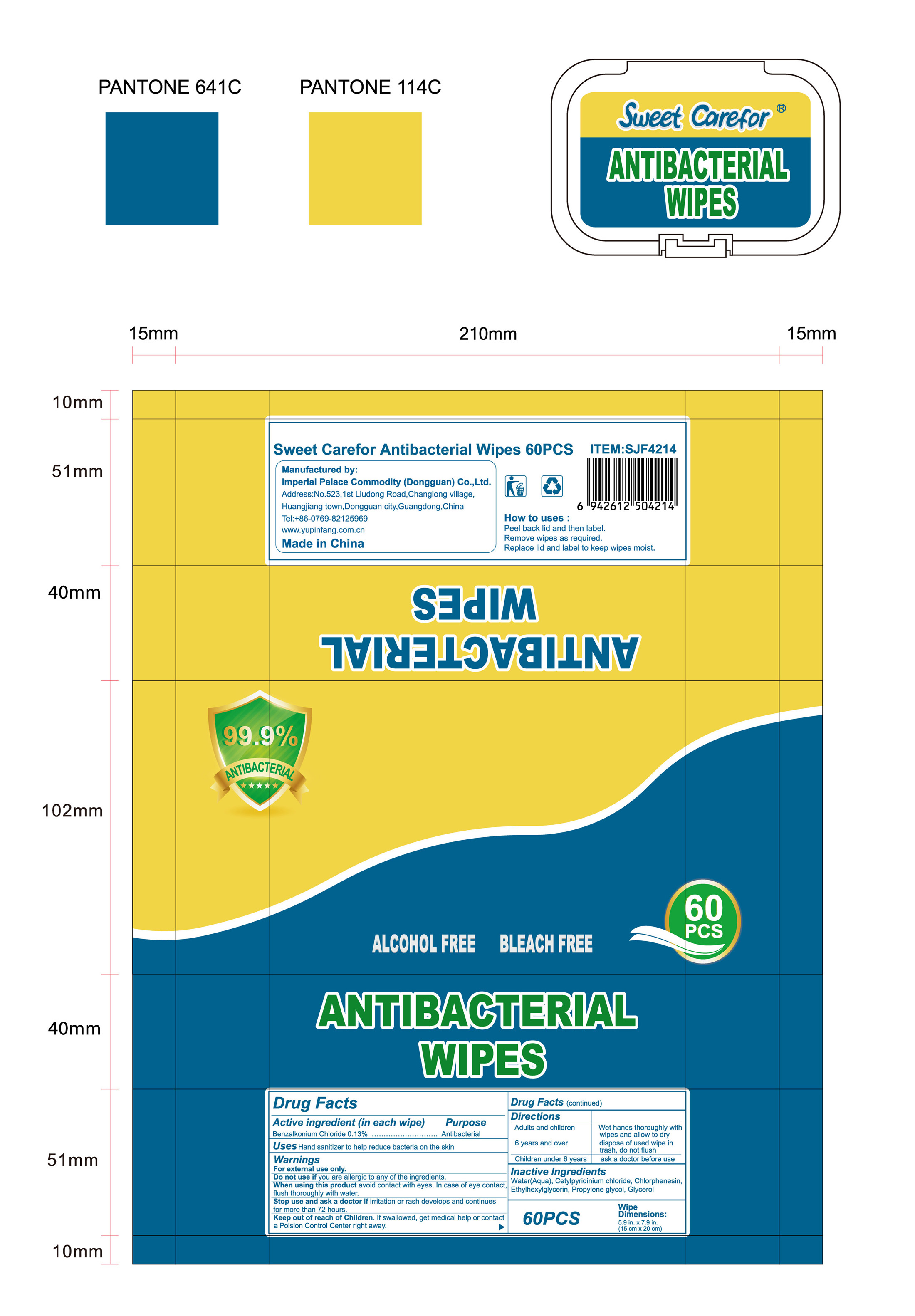
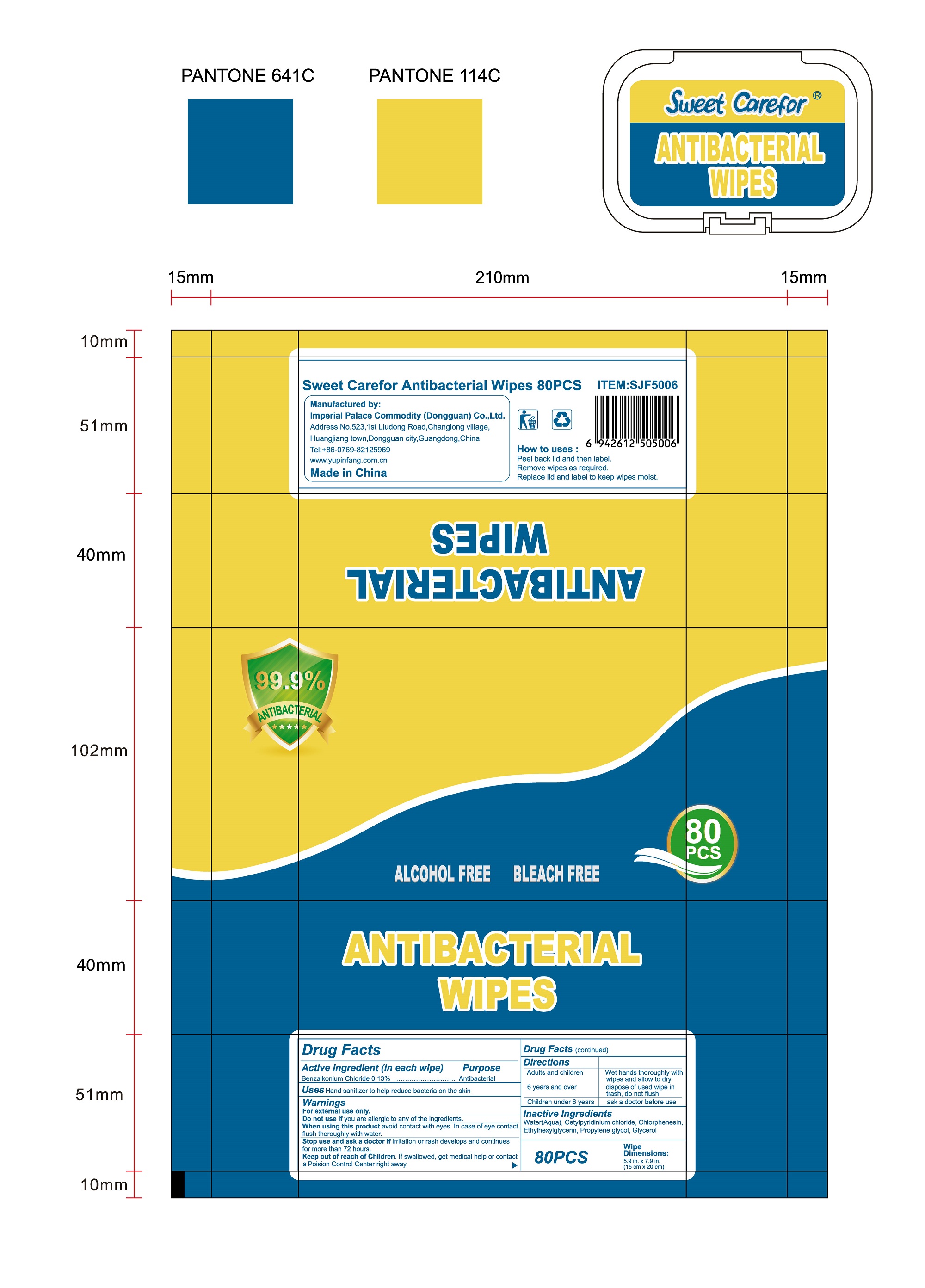
How to uses
Packs
●Peel back lid and then label.
●Remove wipes as required.
●Replace lid and label to keep wipes moist.
Buckets
●Open the bucket lid from the top.
●Locate tear cut in the inner pouch, tear straight to open. Do not remove wipesroll from pouch.
●Pull first wipes from center of roll up through opening.
●Thread first wipe through dispensing nozzle in top of roll.
●Close the bucket lid and dispense the wipes as required.



 NDC:52489-002-06
NDC:52489-002-06
 NDC:52489-002-07
NDC:52489-002-07
 NDC:52489-002-08
NDC:52489-002-08