FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Meclizine hydrochloride tablets are indicated for the treatment of vertigo associated with diseases affecting the vestibular system in adults.
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
- 12.5 mg: light blue colored, oval shaped tablets with “AN 441” debossed on one side and plain on the other side.
- 25 mg: light yellow colored, oval shaped tablets with “AN 442” debossed on one side and plain on the other side.
- 50 mg: white, oval shaped, partially bisected tablets with “AN 444” debossed on one side and plain on the other side.
4 CONTRAINDICATIONS
Meclizine hydrochloride tablets are contraindicated in patients with a hypersensitivity to meclizine or any of the inactive ingredients [see Adverse Reactions (6) and Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Drowsiness
Since drowsiness may occur with use of meclizine hydrochloride, patients should be warned of this possibility and cautioned against driving a car or operating dangerous machinery.
Patients should avoid alcoholic beverages while taking meclizine hydrochloride [see Drug Interactions (7.1)].
6 ADVERSE REACTIONS
The following adverse reactions associated with the use of meclizine hydrochloride were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Anaphylactic reaction, drowsiness, dry mouth, headache, fatigue, and vomiting. On rare occasions blurred vision has been reported.
7 DRUG INTERACTIONS
7.1 CNS Depressants
There may be increased CNS depression when meclizine hydrochloride is administered concurrently with other CNS depressants, including alcohol [see Warnings and Precautions (5.1)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Data from epidemiological studies have not generally indicated a drug-associated risk of major birth defects with meclizine during pregnancy. However, in a published study, an increased incidence of fetal malformations was observed following oral administration of meclizine to pregnant rats during the period of organogenesis, at doses similar to those used clinically. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Human Data
Epidemiological studies reporting on pregnancies exposed to meclizine have not identified an association between the use of meclizine during pregnancy and an increased risk of major birth defects.
Animal Data
In a published study, oral administration of meclizine (25 mg/kg to 250 mg/kg) to pregnant rats during the period of organogenesis resulted in a high incidence of fetal malformations. These effects occurred at doses as low as 25 mg/kg, which is approximately 2 times the maximum recommended human dose (100 mg) on a body surface area (mg/m
2) basis.
8.2 Lactation
Risk Summary
There are no data on the presence of meclizine in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for meclizine hydrochloride and any potential adverse effects on the breastfed infant from meclizine hydrochloride or from the underlying maternal condition.
8.5 Geriatric Use
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of meclizine has not been evaluated. As meclizine hydrochloride undergoes metabolism, hepatic impairment may result in increased systemic exposure of meclizine. Treatment with meclizine hydrochloride should be administered with caution in patients with hepatic impairment.
8.7 Renal Impairment
The effect of renal impairment on the pharmacokinetics of meclizine has not been evaluated. Because of a potential for drug/metabolite accumulation, meclizine hydrochloride should be administered with caution in patients with renal impairment and in the elderly, as renal function generally declines with age.
8.8 Genetic CYP2D6 Polymorphism
The genetic polymorphism of CYP2D6 that results in poor-, intermediate-, extensive-, and ultrarapid metabolizer phenotypes could contribute to large inter-individual variability in meclizine exposure. Therefore, when meclizine hydrochloride is administered to patients with CYP2D6 polymorphism, monitor for adverse reactions and clinical effect accordingly.
11 DESCRIPTION
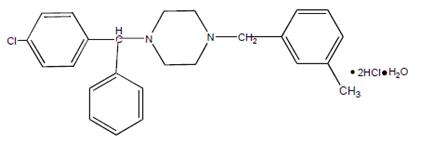
Meclizine hydrochloride, a histamine (H1) receptor antagonist, is a white or slightly yellowish, crystalline powder. It has the following structural formula:
Chemically, meclizine hydrochloride is 1-( p-chloro-α-phenylbenzyl)-4-( m-methylbenzyl) piperazine dihydrochloride monohydrate.
Inactive ingredients for the tablets are: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium starch glycolate and talc. The 12.5 mg tablets also contain FD&C Blue #1 Aluminum Lake. The 25 mg tablets also contain D&C Yellow #10 Aluminum Lake.
Each meclizine hydrochloride 12.5 mg tablet contains 12.5 mg of meclizine dihydrochloride equivalent to 10.53 mg of meclizine free base.
Each meclizine hydrochloride 25 mg tablet contains 25 mg of meclizine dihydrochloride equivalent to 21.07 mg of meclizine free base.
Each meclizine hydrochloride 50 mg tablet contains 50 mg of meclizine dihydrochloride equivalent to 42.14 mg of meclizine free base.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanism by which meclizine exerts its therapeutic effect is unknown but is presumed to involve antagonism of the histamine H1 receptor.
12.3 Pharmacokinetics
The available pharmacokinetic information for meclizine following oral administration has been summarized from published literature.
Absorption
Meclizine is absorbed after oral administration with maximum plasma concentrations reaching at a median T
max value of 3 hours post-dose (range: 1.5 to 6 hours) for the tablet dosage form.
Distribution
Drug distribution characteristics for meclizine in humans are unknown.
Elimination
Meclizine has a plasma elimination half-life of about 5 to 6 hours in humans.
Metabolism
In an
in vitro metabolic study using human hepatic microsome and recombinant CYP enzyme, CYP2D6 was found to be the dominant enzyme for metabolism of meclizine.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Animal studies to assess the carcinogenic potential of meclizine have not been conducted.
Mutagenesis
Genetic toxicology studies of meclizine have not been conducted.
Impairment of Fertility
Animal studies to assess the effects of meclizine on fertility and early embryonic development have not been conducted.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Meclizine Hydrochloride Tablets USP,
12.5 mg are supplied as light blue colored, oval shaped tablets with “AN 441” debossed on one side and plain on the other side.
They are available as follows:
Unit dose packages of 100 (10 x 10) NDC 68084-490-01
Meclizine Hydrochloride Tablets USP,
25 mg are supplied as light yellow colored, oval shaped tablets with “AN 442” debossed on one side and plain on the other side.
They are available as follows:
Unit dose packages of 100 (10 x 10) NDC 68084-491-01
17 PATIENT COUNSELING INFORMATION
Administration Instructions
Advise patients that the tablets must be swallowed whole
[see
Dosage and Administration (2.1)].
Adverse Reactions
Advise patients that meclizine hydrochloride may cause anaphylactic reaction, drowsiness, dry mouth, headache, fatigue, vomiting and, on rare occasions, blurred vision
[see
Warnings and Precautions (5.1),
Adverse Reactions (6)].
Inform patients that meclizine hydrochloride may impair their ability to engage in potentially dangerous activities, such as operating machinery or vehicles.
Concomitant Drug Interactions
Advise patients regarding medications that should not be taken in combination with meclizine hydrochloride or that may necessitate increased monitoring
[see
Drug Interactions (7.1,
7.2)].
Inform patients that alcohol may increase adverse reactions.
Concurrent Medical Conditions
Advise patients to notify their healthcare provider about all of their medical conditions, including if they are pregnant or plan to become pregnant or if they are breastfeeding
[see Warnings and Precautions (5.2), Use in Specific Populations (8.1,
8.2)].
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see
How Supplied section) contain drug product from Amneal Pharmaceuticals, LLC as follows:
(12.5 mg / 100 UD) NDC 68084-490-01 packaged from NDC 65162-441
(25 mg / 100 UD) NDC 68084-491-01 packaged from NDC 65162-442
Distributed by:
American Health Packaging
Columbus, OH 43217
8249001/0919
Package/Label Display Panel – Carton – 12.5 mg

NDC 68084- 490-01
Meclizine
Hydrochloride
Tablets, USP
12.5 mg
100 Tablets (10 x 10) Rx Only
Each Tablet Contains:
12.5 mg meclizine dihydrochloride equivalent to 10.53 mg of
meclizine free base.
Usual Dosage: See package insert for full prescribing information.
VERTIGO: 25 mg to 100 mg in divided doses daily depending on
the clinical response.
Store at 20° to 25°C (68° to 77°F); excursions permitted between
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
NDC # 65162-441, Amneal Pharmaceuticals LLC.
Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217
049001
0249001/0919
Package/Label Display Panel – Carton – 25 mg

NDC 68084- 491-01
Meclizine
Hydrochloride
Tablets, USP
25 mg
100 Tablets (10 x 10) Rx Only
Each Tablet Contains:
25 mg meclizine dihydrochloride equivalent to 21.07 mg of
meclizine free base.
Usual Dosage: See package insert for full prescribing information.
VERTIGO: 25 mg to 100 mg in divided doses daily depending on
the clinical response.
Store at 20° to 25°C (68° to 77°F); excursions permitted between
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
NDC # 65162-442, Amneal Pharmaceuticals LLC.
Distributed by:
American Health Packaging
Columbus, Ohio 43217
049101
0249101/0621