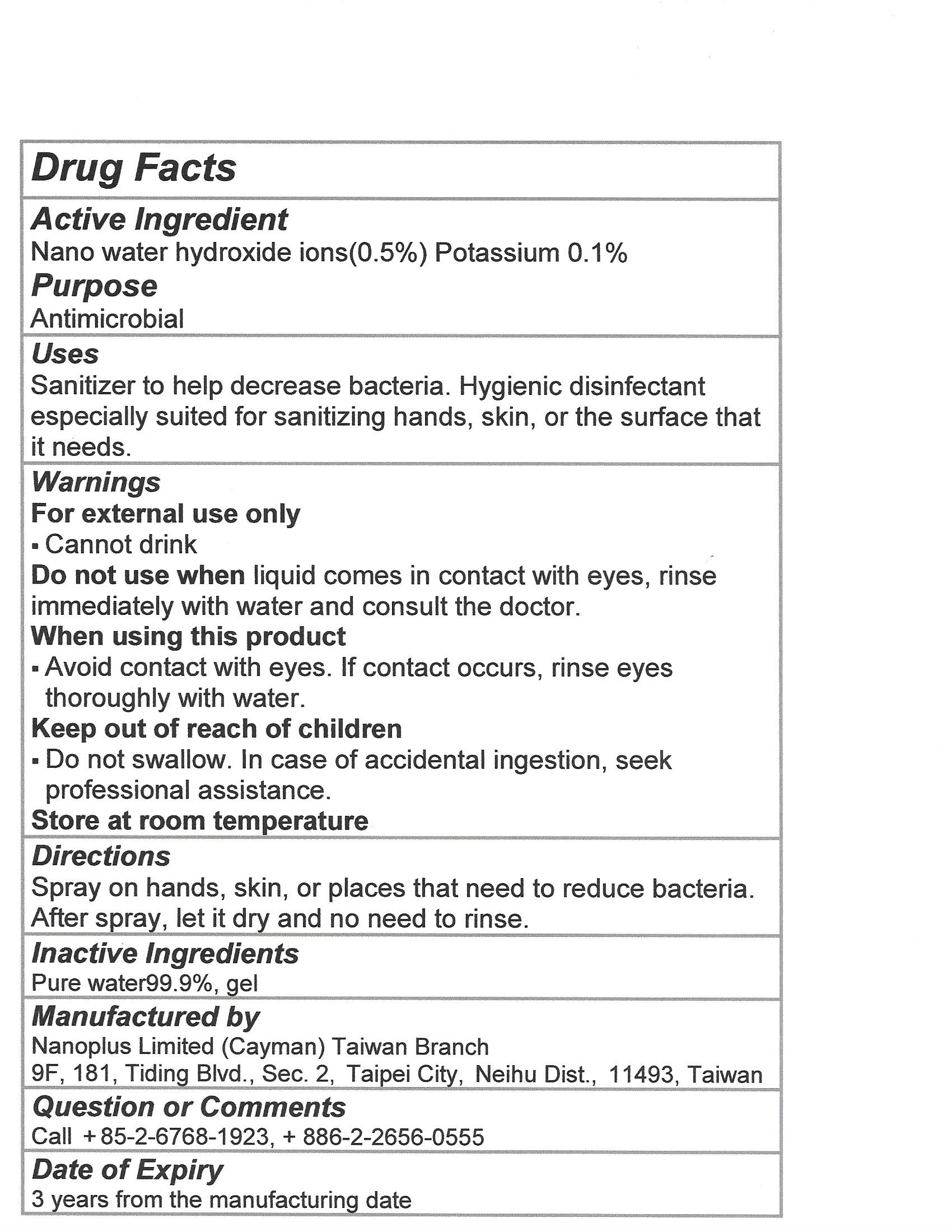
Use
Sanitizer to help decrease bacteria. Hygienic disinfectant especially suited for sanitizing hands, skin or the surface that it needs.
Warnings
For external use only. Cannot drink
Do not use when liquid comes in contact with eyes, rinse immediately with water and consult the doctor
When using this product avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
Keep out of reach of children
Do not swallow. In case of accidental ingestion, seek professional assistance.
Store at room temperature
When using this product avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
Do not use when liquid comes in contact with eyes, rinse immediately with water and consult the doctor
Keep out of reach of children. Do not swallow. In case of accidental ingestion, seek professional assistance.
Keep out of reach of children. Do not swallow. In case of accidental ingestion, seek professional assistance.

 5000 mL NDC: 70970-002-50
5000 mL NDC: 70970-002-50