FULL PRESCRIBING INFORMATION
WARNING: DIFFERENTIATION SYNDROME
Patients treated with IDHIFA have experienced symptoms of differentiation syndrome, which can be fatal if not treated. Symptoms may include fever, dyspnea, acute respiratory distress, pulmonary infiltrates, pleural or pericardial effusions, rapid weight gain or peripheral edema, lymphadenopathy, bone pain, and hepatic, renal, or multi-organ dysfunction. If differentiation syndrome is suspected, initiate corticosteroid therapy and hemodynamic monitoring until symptom resolution [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for the treatment of AML with IDHIFA based on the presence of IDH2 mutations in the blood or bone marrow [see Indications and Usage (1.1) and Clinical Studies (14.1)]. Information on FDA-approved tests for the detection of IDH2 mutations in AML is available at http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
The recommended dosage of IDHIFA is 100 mg taken orally once daily with or without food until disease progression or unacceptable toxicity. For patients without disease progression or unacceptable toxicity, treat for a minimum of 6 months to allow time for clinical response.
Swallow tablets whole. Do not chew, split, or crush IDHIFA tablets. Administer IDHIFA tablets orally about the same time each day. If a dose of IDHIFA is vomited, missed, or not taken at the usual time, administer the dose as soon as possible on the same day, and return to the normal schedule the following day.
2.3 Monitoring and Dosage Modifications for Toxicities
Assess blood counts and blood chemistries for leukocytosis and tumor lysis syndrome prior to the initiation of IDHIFA and monitor at a minimum of every 2 weeks for at least the first 3 months during treatment. Manage any abnormalities promptly [see Adverse Reactions (6.1)].
Interrupt dosing or reduce dose for toxicities. See Table 1 for dosage modification guidelines.
| *Grade 1 is mild, Grade 2 is moderate, Grade 3 is serious, Grade 4 is life-threatening. | |
|
Adverse Reaction |
Recommended Action |
|
|
|
|
|
|
|
|
3 DOSAGE FORMS AND STRENGTHS
IDHIFA is available in the following tablet strengths:
- •
- 50 mg enasidenib tablet: Pale yellow to yellow oval-shaped film-coated tablet debossed "ENA" on one side and "50" on the other side.
- •
- 100 mg enasidenib tablet: Pale yellow to yellow capsule-shaped film-coated tablet debossed "ENA" on one side and "100" on the other side.
5 WARNINGS AND PRECAUTIONS
5.1 Differentiation Syndrome
In the clinical trial, 14% of patients treated with IDHIFA experienced differentiation syndrome, which may be life-threatening or fatal if not treated. Differentiation syndrome is associated with rapid proliferation and differentiation of myeloid cells. While there is no diagnostic test for differentiation syndrome, symptoms in patients treated with IDHIFA included acute respiratory distress represented by dyspnea and/or hypoxia (68%) and need for supplemental oxygen (76%); pulmonary infiltrates (73%) and pleural effusion (45%); renal impairment (70%); fever (36%); lymphadenopathy (33%); bone pain (27%); peripheral edema with rapid weight gain (21%); and pericardial effusion (18%). Hepatic, renal, and multi-organ dysfunction have also been observed.
Differentiation syndrome has been observed with and without concomitant hyperleukocytosis, in as early as 1 day and up to 5 months after IDHIFA initiation.
If differentiation syndrome is suspected, initiate oral or intravenous corticosteroids (e.g., dexamethasone 10 mg every 12 hours) and hemodynamic monitoring until improvement. Taper corticosteroids only after resolution of symptoms. Symptoms of differentiation syndrome may recur with premature discontinuation of corticosteroid treatment. If severe pulmonary symptoms requiring intubation or ventilator support, and/or renal dysfunction persist for more than 48 hours after initiation of corticosteroids, interrupt IDHIFA until signs and symptoms are no longer severe [see Dosage and Administration (2.3)]. Hospitalization for close observation and monitoring of patients with pulmonary and/or renal manifestation is recommended.
5.2 Embryo-Fetal Toxicity
Based on animal embryo-fetal toxicity studies, IDHIFA can cause embryo-fetal harm when administered to a pregnant woman. In animal embryo-fetal toxicity studies, enasidenib caused embryo-fetal toxicities starting at 0.1 times the steady state clinical exposure based on the area under the concentration-time curve (AUC) at the recommended human dose.
Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective non‑hormonal contraception during treatment with IDHIFA and for 2 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with IDHIFA and for 2 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Differentiation Syndrome [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety evaluation of single-agent IDHIFA is based on 214 patients with relapsed or refractory AML who were assigned to receive 100 mg daily [see Clinical Studies (14.1)]. The median duration of exposure to IDHIFA was 4.3 months (range 0.3 to 23.6). The 30-day and 60-day mortality rates observed with IDHIFA were 4.2% (9/214) and 11.7% (25/214), respectively.
Serious adverse reactions were reported in 77.1% of patients. The most frequent serious adverse reactions (≥2%) were leukocytosis (10%), diarrhea (6%), nausea (5%), vomiting (3%), decreased appetite (3%), tumor lysis syndrome (5%), and differentiation syndrome (8%). Differentiation syndrome events characterized as serious included pyrexia, renal failure acute, hypoxia, respiratory failure, and multi-organ failure.
Overall, 92 of 214 patients (43%) required a dose interruption due to an adverse reaction; the most frequent adverse reactions leading to dose interruption were differentiation syndrome (4%) and leukocytosis (3%). Ten of 214 patients (5%) required a dose reduction due to an adverse reaction; no adverse reaction required dose reduction in more than 2 patients. Thirty-six of 214 patients (17%) permanently discontinued IDHIFA due to an adverse reaction; the most frequent adverse reaction leading to permanent discontinuation was leukocytosis (1%).
The most common adverse reactions (≥20%) of any grade were nausea, vomiting, diarrhea, elevated bilirubin and decreased appetite.
Adverse reactions reported in the trial are shown in Table 2.
| a Gastrointestinal disorders observed with IDHIFA treatment can be associated with other commonly reported events such as abdominal pain, and weight decreased. b Tumor lysis syndrome observed with IDHIFA treatment can be associated with commonly reported uric acid increased. c Differentiation syndrome can be associated with other commonly reported events such as respiratory failure, dyspnea, hypoxia, pyrexia, peripheral edema, rash, or renal insufficiency. |
||
|
IDHIFA (100 mg daily) |
||
|
Body System
|
All Grades |
≥Grade 3 |
|
Gastrointestinal Disorders a |
||
|
Nausea |
107 (50) |
11 (5) |
|
Diarrhea |
91 (43) |
17 (8) |
|
Vomiting |
73 (34) |
4 (2) |
|
Metabolism and Nutrition Disorders |
||
|
Decreased appetite |
73 (34) |
9 (4) |
|
Tumor lysis syndrome b |
13 (6) |
12 (6) |
|
Blood and Lymphatic System Disorders |
||
|
Differentiation syndrome c |
29 (14) |
15 (7) |
|
Noninfectious leukocytosis |
26 (12) |
12 (6) |
|
Nervous System Disorders |
||
|
Dysgeusia |
25 (12) |
0 (0) |
Other clinically significant adverse reactions occurring in <10% of patients included:
- •
- Respiratory, Thoracic, and Mediastinal Disorders: Pulmonary edema, acute respiratory distress syndrome
Changes in selected post-baseline laboratory values that were observed in patients with relapsed or refractory AML are shown in Table 3.
| IDHIFA (100 mg daily) N=214 |
||
|---|---|---|
| Parameter a | All Grades (%) | Grade ≥3 (%) |
| a Includes abnormalities occurring up to 28 days after last IDHIFA dose, if new or worsened by at least one grade from baseline, or if baseline was unknown. The denominator varies based on data collected for each parameter (N=213 except phosphorous N=209). | ||
|
Total bilirubin increased |
81 |
15 |
|
Calcium decreased |
74 |
8 |
|
Potassium decreased |
41 |
15 |
|
Phosphorus decreased |
27 |
8 |
Elevated Bilirubin
IDHIFA may interfere with bilirubin metabolism through inhibition of UGT1A1 [see Clinical Pharmacology (12.3)]. Thirty-seven percent of patients (80/214) experienced total bilirubin elevations ≥2 x ULN at least one time. Of those patients who experienced total bilirubin elevations ≥2 x ULN, 35% had elevations within the first month of treatment, and 89% had no concomitant elevation of transaminases or other severe adverse events related to liver disorders. No patients required a dose reduction for hyperbilirubinemia; treatment was interrupted in 3.7% of patients, for a median of 6 days. Three patients (1.4%) discontinued IDHIFA permanently due to hyperbilirubinemia.
7 DRUG INTERACTIONS
7.1 Effect of IDHIFA on Other Drugs
Certain CYP1A2 Substrates
Avoid concomitant use with IDHIFA unless otherwise recommended in the Prescribing Information for CYP1A2 substrates where minimal concentration changes may lead to serious adverse reactions. Consider reducing the frequency of caffeine intake from various food and beverages in a 24 hour period while taking IDHIFA because IDHIFA may increase the effect of caffeine in patients who are sensitive to it.
Enasidenib is a CYP1A2 inhibitor. Concomitant use of IDHIFA increases the exposure of CYP1A2 substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions related to the substrates.
Certain CYP2C19 substrates
Avoid concomitant use with IDHIFA unless otherwise recommended in the Prescribing Information for CYP2C19 substrates where minimal concentration changes may lead to serious adverse reactions.
Enasidenib is a CYP2C19 inhibitor. Concomitant use of IDHIFA increases the exposure of CYP2C19 substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions related to these substrates.
Certain CYP3A substrates
Avoid concomitant use with IDHIFA unless otherwise recommended in the Prescribing Information for CYP3A substrates where minimal concentration changes may lead to reduced efficacy.
Do not administer IDHIFA with anti-fungal agents that are substrates of CYP3A due to expected loss of antifungal efficacy.
Co-administration of IDHIFA may decrease the concentrations of hormonal contraceptives. Consider alternative methods of contraception in patients receiving IDHIFA [See use in Specific Population (8.1, 8.3)].
Enasidenib is a CYP3A inducer. Concomitant use of IDHIFA decreases the exposure of CYP3A substrates [see Clinical Pharmacology (12.3)], which may reduce the efficacy of the substrates.
Certain OATP1B1, OATP1B3, and BCRP Substrates
Avoid coadministration of IDHIFA with OATP1B1, OATP1B3, and BCRP substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the OATP1B1, OATP1B3, and BCRP substrates dosage(s) in accordance with the respective Prescribing Information.
Enasidenib is an OATP1B1, OATP1B3, and BCRP transporter inhibitor. Concomitant use of IDHIFA increases the exposure of OATP1B1, OATP1B3, and BCRP substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions related to these substrates.
Certain P-glycoprotein (P-gp) Substrates
When coadministered with IDHIFA, follow recommended P-gp substrates Prescribing Information and monitor more frequently for adverse reactions related to these substrates.
Enasidenib is a P-gp transporter inhibitor. Concomitant use of IDHIFA increases the exposure of P-gp substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions related to the substrates.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal embryo-fetal toxicity studies, IDHIFA can cause fetal harm when administered to a pregnant woman. There are no available data on IDHIFA use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. In animal embryo-fetal toxicity studies, oral administration of enasidenib to pregnant rats and rabbits during organogenesis was associated with embryo-fetal mortality and alterations to growth starting at 0.1 times the steady state clinical exposure based on the AUC at the recommended human dose (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data
Animal Data
Enasidenib administered to pregnant rats at a dose of 30 mg/kg twice daily during organogenesis (gestation days 6-17) was associated with maternal toxicity and adverse embryo-fetal effects including post-implantation loss, resorptions, decreased viable fetuses, lower fetal birth weights, and skeletal variations. These effects occurred in rats at approximately 1.6 times the clinical exposure at the recommended human daily dose of 100 mg/day.
In pregnant rabbits treated during organogenesis (gestation days 7-19), enasidenib was maternally toxic at doses equal to 5 mg/kg/day or higher (exposure approximately 0.1 to 0.6 times the steady state clinical exposure at the recommended daily dose) and caused spontaneous abortions at 5 mg/kg/day (exposure approximately 0.1 times the steady state clinical exposure at the recommended daily dose).
8.2 Lactation
Risk Summary
There are no data on the presence of enasidenib or its metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for adverse reactions in the breastfed child, advise women not to breastfeed during treatment with IDHIFA and for 2 months after the last dose.
8.3 Females and Males of Reproductive Potential
Based on animal embryo-fetal toxicity studies, IDHIFA can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to starting IDHIFA.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with IDHIFA and for 2 months after the last dose. Coadministration of IDHIFA may decrease the concentrations of combined hormonal contraceptives. Advise patients using hormonal contraceptives to use an effective non-hormonal contraceptive method during treatment with IDHIFA and for 2 months after the last dose [see Drug Interactions (7.1)].
Infertility
Based on findings in animals, IDHIFA may impair fertility in females and males of reproductive potential. It is not known whether these effects on fertility are reversible [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of IDHIFA in pediatric patients have not been established.
8.5 Geriatric Use
No dosage adjustment is required for IDHIFA based on age. In the clinical study, 61% of 214 patients were aged 65 years or older, while 24% were older than 75 years. No overall differences in effectiveness or safety were observed between patients aged 65 years or older and younger patients.
11 DESCRIPTION
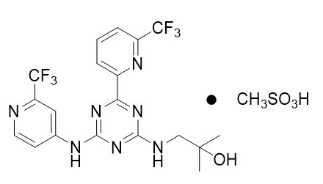
Enasidenib is an inhibitor of isocitrate dehydrogenase-2 (IDH2) enzyme. Enasidenib is available as the mesylate salt with the chemical name:
2-methyl-1-[(4-[6-(trifluoromethyl)pyridin-2-yl]-6-{[2-(trifluoromethyl)pyridin-4-yl]amino}-1,3,5-triazin-2-yl)amino]propan-2-ol methanesulfonate.
Or
2-Propanol, 2-methyl-1-[[4-[6-(trifluoromethyl)-2-pyridinyl]-6-[[2-(trifluoromethyl)-4-pyridinyl]amino-1,3,5-triazin-2-yl]amino]-, methanesulfonate (1:1).
The chemical structure is:
The empirical formula is C19H17F6N7O ∙ CH3SO3H (C20H21F6N7O4S), and the molecular weight is 569.48 g/mol. Enasidenib is practically insoluble (solubility ≤74 mcg/mL) in aqueous solutions across physiological pH range (pH 1.2 and 7.4).
IDHIFA (enasidenib) is available as a 50 mg tablet (equivalent to 60 mg enasidenib mesylate) and a 100 mg tablet (equivalent to 120 mg enasidenib mesylate) for oral administration. Each tablet contains inactive ingredients of colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose acetate succinate, iron oxide yellow, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, sodium lauryl sulfate, sodium starch glycolate, talc, and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Enasidenib is a small molecule inhibitor of the isocitrate dehydrogenase 2 (IDH2) enzyme. Enasidenib targets the mutant IDH2 variants R140Q, R172S, and R172K at approximately 40-fold lower concentrations than the wild-type enzyme in vitro. Inhibition of the mutant IDH2 enzyme by enasidenib led to decreased 2-hydroxyglutarate (2-HG) levels and induced myeloid differentiation in vitro and in vivo in mouse xenograft models of IDH2 mutated AML. In blood samples from patients with AML with mutated IDH2, enasidenib decreased 2-HG levels, reduced blast counts and increased percentages of mature myeloid cells.
12.3 Pharmacokinetics
The peak plasma concentration (Cmax) is 1.4 mcg/mL [coefficient of variation (CV%) 50%] after a single dose of 100 mg, and 13.1 mcg/mL (CV% 45%) at steady state for 100 mg daily. The area under concentration time curve (AUC) of enasidenib increases in an approximately dose proportional manner from 50 mg (0.5 times approved recommended dosage) to 450 mg (4.5 times approved recommended dosage) daily dose. Steady-state plasma levels are reached within 29 days of once-daily dosing. Accumulation is approximately 10-fold when administered once daily.
Absorption
The absolute bioavailability after 100 mg oral dose of enasidenib is approximately 57%. After a single oral dose, the median time to Cmax (Tmax) is 4 hours.
Distribution
The mean volume of distribution (Vd) of enasidenib is 55.8 L (CV% 29). Human plasma protein binding of enasidenib is 98.5% and of its metabolite AGI-16903 is 96.6% in vitro.
Elimination
Enasidenib has a terminal half-life of 7.9 days and a mean total body clearance (CL/F) of 0.70 L/hour (CV% 62.5).
Metabolism
Enasidenib accounted for 89% of the radioactivity in circulation and AGI-16903, the N-dealkylated metabolite, represented 10% of the circulating radioactivity.
Metabolism of enasidenib is mediated by multiple cytochrome P450 (CYP) enzymes (e.g., CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4), and by multiple UDP glucuronosyl transferases (UGTs) (e.g., UGT1A1, UGT1A3, UGT1A4, UGT1A9, UGT2B7, and UGT2B15) in vitro. Further metabolism of the metabolite AGI-16903 is also mediated by multiple enzymes (e.g., CYP1A2, CYP2C19, CYP3A4, UGT1A1, UGT1A3, and UGT1A9) in vitro.
Specific Populations
No clinically meaningful effect on the pharmacokinetics of enasidenib was observed for the following covariates: age (19 years to 100 years), race (White, Black, or Asian), mild hepatic impairment [defined as total bilirubin ≤ upper limit of normal (ULN) and aspartate transaminase (AST) >ULN or total bilirubin 1 to 1.5 times ULN and any AST], renal impairment (defined as creatinine clearance ≥30 mL/min by Cockcroft-Gault formula), sex, body weight (39 kg to 136 kg), and body surface area.
Drug Interaction Studies
Clinical Studies
CYP1A2 Substrates: Caffeine (a sensitive CYP1A2 substrate) AUC0-INF and Cmax increased by 655% and 18%, respectively, following concomitant use of multiple doses of IDHIFA 100 mg with a single oral dose of 100 mg caffeine.
CYP2C19 substrates: Omeprazole (a sensitive CYP2C19 substrate) AUC0-INF and Cmax increased by 86% and 47%, respectively, following concomitant use of multiple doses of IDHIFA 100 mg with a single oral dose of omeprazole 40 mg.
CYP2D6 substrates: Dextromethorphan (a sensitive CYP2D6 substrate) AUC0-INF and Cmax increased by 37% and 24% respectively, following concomitant use of multiple doses of IDHIFA 100 mg with a single oral dose of dextromethorphan 30 mg.
CYP3A substrates: Midazolam (a sensitive CYP3A substrate) AUC0‑INF and Cmax decreased by 43% and 23%, respectively, following concomitant use of multiple doses of IDHIFA 100 mg with a single intravenous dose of midazolam 0.03 mg/kg. The decrease in midazolam exposure following oral administration of midazolam is expected to be larger than that following intravenous administration of midazolam. This is due to induction of CYP3A4 first pass metabolism by enasidenib and the reduced bioavailability of the midazolam oral formulation compared to the midazolam intravenous formulation.
CYP2C9 and CYP2C8 substrates: Coadministration of multiple doses of IDHIFA 100 mg did not have clinically significant effect on the exposure of flurbiprofen (a CYP2C9 substrate) or pioglitazone (a CYP2C8 substrate).
OATP1B1, OATP1B3, and BCRP Substrates: Rosuvastatin (an OATP1B1, OATP1B3, and BCRP substrate) AUC0-INF and Cmax increased by 244% and 366%, respectively, following concomitant use of multiple doses of IDHIFA 100 mg with a single oral dose of rosuvastatin 10 mg.
P-gp Substrates: Digoxin (a P-gp Substrate) AUC0-30h and Cmax increased by 20% and 26%, respectively, following concomitant use of multiple doses of IDHIFA 100 mg with a single oral dose of digoxin 0.25 mg.
In Vitro Studies
CYP and UGT Enzymes: Enasidenib inhibits CYP2B6 and UGT1A1. The metabolite AGI-16903 inhibits CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2D6. Enasidenib induces CYP2B6.
Transporter Systems: Enasidenib is not a substrate for P-glycoprotein (P-gp) or breast cancer resistance protein (BCRP). AGI-16903 is a substrate of both P-gp and BCRP. Enasidenib and AGI-16903 are not substrates of multidrug resistance-associated protein 2 (MRP2), organic anion transporter 1 (OAT1), OAT3, organic anion transporter family member 1B1 (OATP1B1), OATP1B3, and organic cation transporter 2 (OCT2).
Enasidenib inhibits OAT1 and OCT2, but not MRP2 or OAT3. AGI-16903 inhibits BCRP, OAT1, OAT3, OATP1B1, and OCT2, but not P-gp, MRP2, or OATP1B3.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with enasidenib.
Enasidenib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay. Enasidenib was not clastogenic in an in vitro human lymphocyte chromosomal aberration assay, or in an in vivo rat bone marrow micronucleus assay.
Fertility studies in animals have not been conducted with enasidenib. In repeat-dose toxicity studies with twice daily oral administration of enasidenib in rats up to 90-days in duration, changes were reported in male and female reproductive organs including seminiferous tubular degeneration, hypospermia, atrophy of the seminal vesicle and prostate, decreased corpora lutea and increased atretic follicles in the ovaries, and atrophy in the uterus.
14 CLINICAL STUDIES
14.1 Acute Myeloid Leukemia
The efficacy of IDHIFA was evaluated in an open-label, single-arm, multicenter, two-cohort clinical trial (Study AG221-C-001, NCT01915498) of 199 adult patients with relapsed or refractory AML and an IDH2 mutation, who were assigned to receive 100 mg daily dose. Cohort 1 included 101 patients and Cohort 2 included 98 patients. IDH2 mutations were identified by a local diagnostic test and retrospectively confirmed by the Abbott RealTime IDH2 assay, or prospectively identified by the Abbott RealTime IDH2 assay, which is the FDA-approved test for selection of patients with AML for treatment with IDHIFA. IDHIFA was given orally at starting dose of 100 mg daily until disease progression or unacceptable toxicity. Dose reductions were allowed to manage adverse events.
The baseline demographic and disease characteristics are shown in Table 4. The baseline demographics and disease characteristics were similar in both study cohorts.
| Demographic and Disease Characteristics | IDHIFA (100 mg daily) N=199 |
|---|---|
| Demographics | |
| ECOG PS: Eastern Cooperative Oncology Group Performance Status. a 1 patient had missing baseline ECOG PS. b For 3 patients with different mutations detected in bone marrow compared to blood, the result of blood is reported. c Patients were defined as transfusion dependent at baseline if they received any red blood cell or platelet transfusions within the 8-week baseline period. d Includes intensive and/or nonintensive therapies. |
|
|
Age (Years) Median (Min, Max) |
68 (19, 100) |
|
Age Categories, n (%) | |
|
<65 years |
76 (38) |
|
≥65 years to <75 years |
74 (37) |
|
≥75 years |
49 (25) |
|
Sex, n (%) | |
|
Male |
103 (52) |
|
Female |
96 (48) |
|
Race, n (%) | |
|
White |
153 (77) |
|
Black |
10 (5) |
|
Asian |
1 (1) |
|
Native Hawaiian/Other Pacific Islander |
1 (1) |
|
Other / Not Provided |
34 (17) |
|
Disease Characteristics, n (%) | |
|
ECOG PS a, n (%) | |
|
0 |
46 (23) |
|
1 |
124 (62) |
|
2 |
28 (14) |
|
Relapsed AML, n (%) |
95 (48) |
|
Refractory AML, n (%) |
104 (52) |
|
IDH2 Mutation b, n (%) | |
|
R140 |
155 (78) |
|
R172 |
44 (22) |
|
Time from Initial AML Diagnosis (months) | |
|
Median (min, max) (172 patients) |
11.3 (1.2, 129.1) |
|
Cytogenetic Risk Status, n (%) | |
|
Intermediate |
98 (49) |
|
Poor |
54 (27) |
|
Missing /Failure |
47 (24) |
|
Prior Stem Cell Transplantation for AML, n (%) |
25 (13) |
|
Transfusion Dependent at Baseline c, n (%) |
157 (79) |
|
Number of Prior Anticancer Regimens, n (%) d | |
|
1 |
89 (45) |
|
2 |
64 (32) |
|
≥3 |
46 (23) |
|
Median number of prior therapies (min, max) |
2 (1, 6) |
Efficacy was established on the basis of the rate of complete response (CR)/complete response with partial hematologic recovery (CRh), the duration of CR/CRh, and the rate of conversion from transfusion dependence to transfusion independence. The efficacy results are shown in Table 5 and were similar in both cohorts. The median follow-up was 6.6 months (range, 0.4 to 27.7 months). Similar CR/CRh rates were observed in patients with either R140 or R172 mutation.
| Endpoint | IDHIFA (100 mg daily) N=199 |
|---|---|
| CI: confidence interval, NA: not available. a CR (complete remission) was defined as <5% of blasts in the bone marrow, no evidence of disease, and full recovery of peripheral blood counts (platelets >100,000/microliter and absolute neutrophil counts [ANC] >1,000/microliter). b DOR (duration of response) was defined as time since first response of CR or CRh to relapse or death, whichever is earlier. c CRh (complete remission with partial hematological recovery) was defined as <5% of blasts in the bone marrow, no evidence of disease, and partial recovery of peripheral blood counts (platelets >50,000/microliter and ANC >500/microliter). |
|
|
CRa n (%) |
37 (19) |
|
95% CI |
(13, 25) |
|
Median DORb (months) |
8.2 |
|
95% CI |
(4.7, 19.4) |
|
CRhc n (%) |
9 (4) |
|
95% CI |
(2, 8) |
|
Median DOR (months) |
9.6 |
|
95% CI |
(0.7, NA) |
|
CR/CRh n (%) |
46 (23) |
|
95% CI |
(18, 30) |
|
Median DOR (months) |
8.2 |
|
95% CI |
(4.3, 19.4) |
For patients who achieved a CR/CRh, the median time to first response was 1.9 months (range, 0.5 to 7.5 months) and the median time to best response of CR/CRh was 3.7 months (range, 0.6 to 11.2 months). Of the 46 patients who achieved a best response of CR/CRh, 39 (85%) did so within 6 months of initiating IDHIFA.
Among the 157 patients who were dependent on red blood cell (RBC) and/or platelet transfusions at baseline, 53 (34%) became independent of RBC and platelet transfusions during any 56-day post baseline period. Of the 42 patients who were independent of both RBC and platelet transfusions at baseline, 32 (76%) remained transfusion independent during any 56-day post baseline period.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
50 mg tablet: Pale yellow to yellow oval-shaped film-coated tablet debossed “ENA” on one side and “50” on the other side.
- •
- 30-count bottles of 50 mg tablets with a desiccant canister (NDC 59572-705-30)
100 mg tablet: Pale yellow to yellow capsule-shaped film-coated tablet debossed “ENA” on one side and “100” on the other side.
- •
- 30-count bottles of 100 mg tablets with a desiccant canister (NDC 59572-710-30)
Storage
Store at 20°C-25°C (68°F-77°F); excursions permitted between 15°C-30°C (59°F-86°F) [see USP Controlled Room Temperature]. Keep the bottle tightly closed. Store and dispense in the original bottle (with a desiccant canister) to protect from moisture.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Differentiation Syndrome
Advise patients on the risks of developing differentiation syndrome as early as 1 day and during the first 5 months on treatment. Ask patients to immediately report any symptoms suggestive of differentiation syndrome, such as fever, cough or difficulty breathing, bone pain, rapid weight gain or swelling of their arms or legs, to their healthcare provider for further evaluation [see Boxed Warning and Warnings and Precautions (5.1)].
Tumor Lysis Syndrome
Advise patients on the risks of developing tumor lysis syndrome. Advise patients on the importance of maintaining high fluid intake and the need for frequent monitoring of blood chemistry values [see Dosage and Administration (2.3) and Adverse Reactions (6.1)].
Gastrointestinal Adverse Reactions
Advise patients on risk of experiencing gastrointestinal reactions, such as diarrhea, nausea, vomiting, decreased appetite, and changes in their sense of taste. Ask patients to report these reactions to their healthcare provider, and advise patients how to manage them [see Adverse Reactions (6.1)].
Elevated Blood Bilirubin
Inform patients that taking IDHIFA may cause elevated blood bilirubin, which is due to its mechanism of action, and not due to liver damage. Advise patients to report any changes to the color of their skin or the whites of their eyes to their healthcare provider for further evaluation [see Adverse Reactions (6.1)].
Embryo-Fetal Toxicity
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to notify their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.2), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use an effective non-hormonal contraceptive method during treatment with IDHIFA and for 2 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with IDHIFA and for 2 months after the last dose. Coadministration of IDHIFA may decrease the concentrations of combined hormonal contraceptives [see Drug Interactions (7.1) and Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with IDHIFA and for 2 months after the last dose [see Use in Specific Populations (8.2)].
Drug Interactions
Advise patients to inform their healthcare providers of all concomitant products, including over-the-counter products and supplements [see Drug Interactions (7.1)].
Dosing and Storage Instructions
- •
- Advise patients not to chew, split, or crush the tablets but swallow whole with a cup of water.
- •
- Instruct patients that if they miss a dose or vomit after a dose of IDHIFA, to take it as soon as possible on the same day and return to normal schedule the following day. Advise patients not to take 2 doses to make up for the missed dose [see Dosage and Administration (2.2)].
- •
- Keep IDHIFA in the original container. Keep the container tightly closed with desiccant canister inside to protect the tablets from moisture [see How Supplied/Storage and Handling (16)].
Marketed by:
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
Licensed from:
Servier Pharmaceuticals LLC
Boston, MA 02210
Trademarks are the property of their respective owners.
IDHIFA® is a trademark of Celgene Corporation, a Bristol Myers Squibb company.
Pat. https://www.bms.com/patient-and-caregivers/our-medicines.html
IDHPI.006/MG.006
|
MEDICATION GUIDE IDHIFA® (eyed-HEE-fuh) (enasidenib) tablets |
||
|
What is the most important information I should know about IDHIFA? IDHIFA may cause serious side effects, including:
|
||
|
|
|
|
If you develop any of these symptoms of differentiation syndrome, your healthcare provider may start you on a medicine taken by mouth or given through a vein (intravenous) called corticosteroids and may monitor you in the hospital. |
||
|
What is IDHIFA? IDHIFA is a prescription medicine used to treat people with acute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation whose disease has come back or has not improved after previous treatment(s). It is not known if IDHIFA is safe and effective in children. |
||
|
Before taking IDHIFA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
||
|
How should I take IDHIFA?
|
||
|
What are the possible side effects of IDHIFA? IDHIFA may cause serious side effects, including: See “What is the most important information I should know about IDHIFA?” The most common side effects of IDHIFA include: |
||
|
|
|
|
Tell your healthcare provider if you have any changes to the color of your skin or the whites of your eyes. Your healthcare provider will monitor you for side effects during treatment and may tell you to stop taking IDHIFA if you develop certain side effects. These are not all the possible side effects of IDHIFA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
How should I store IDHIFA?
Keep IDHIFA and all medicines out of the reach of children. |
||
|
General information about the safe and effective use of IDHIFA Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take IDHIFA for conditions for which it was not prescribed. Do not give IDHIFA to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about IDHIFA that is written for health professionals. |
||
|
What are the ingredients in IDHIFA? Active ingredient: enasidenib Inactive ingredients: colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose acetate succinate, iron oxide yellow, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, sodium lauryl sulfate, sodium starch glycolate, talc, and titanium dioxide Marketed by: Bristol-Myers Squibb Company, Princeton, NJ 08543 USA Licensed from: Servier Pharmaceuticals LLC, Boston, MA 02210 IDHIFA® is a trademark of Celgene Corporation, a Bristol Myers Squibb company. Pat. https://www.bms.com/patient-and-caregivers/our-medicines.html IDHMG.006 For more information go to www.IDHIFA.com or call 1-800-721-5072. |
||
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 12/2023