ZYMAR- gatifloxacin solution/ drops
Allergan, Inc.
----------
Zymar
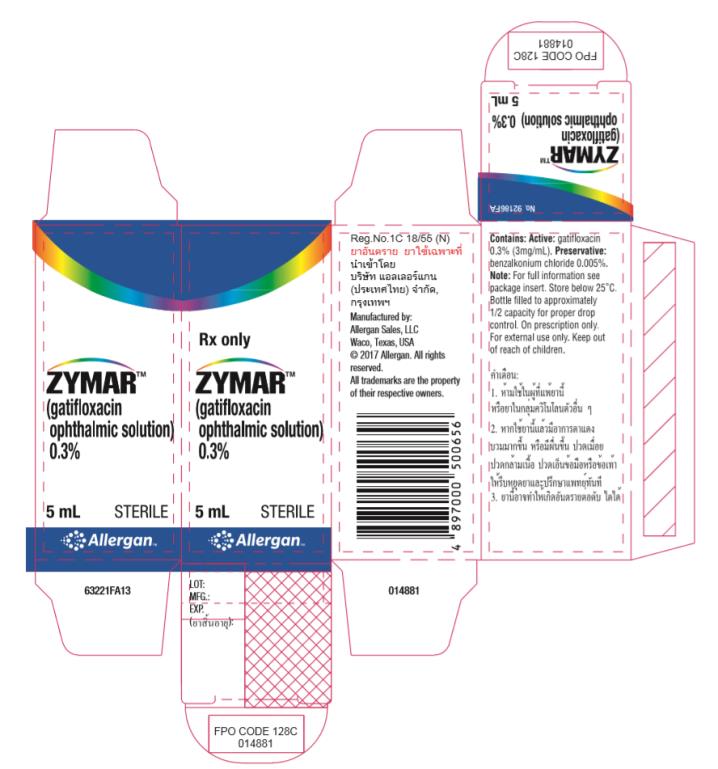
PRINCIPAL DISPLAY PANEL - 3 MG/ML CARTON LABEL
Rx only
ZYMARTM
(gatifloxacin
ophthalmic solution)
0.3%
5 mL STERILE
® ALLERGAN
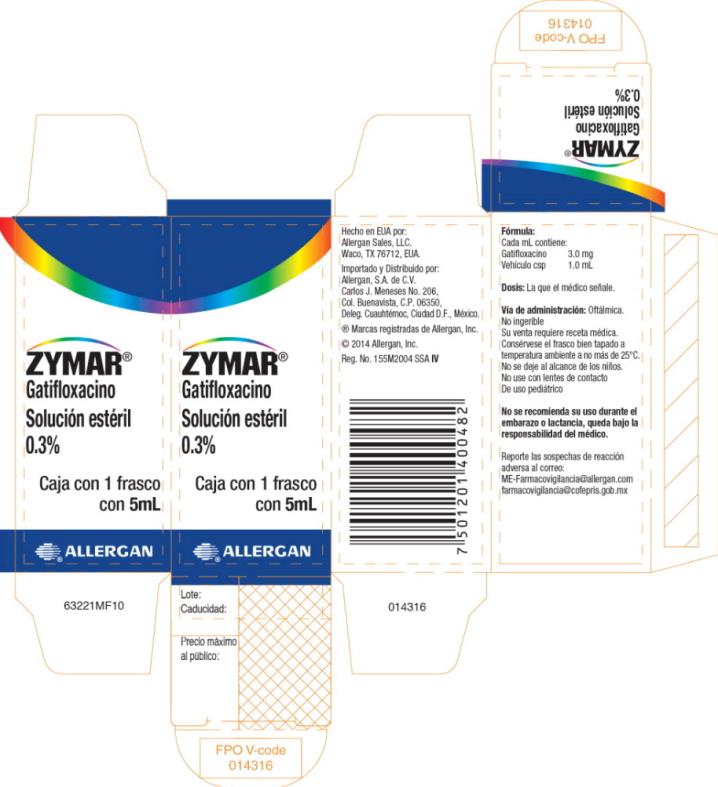
PRINCIPAL DISPLAY PANEL - 3 MG/ML CARTON LABEL
ZYMAR®
Gatifloxacino
Solución estéril
0.3%
Caja con 1 frasco
con 5mL
®ALLERGAN
| ZYMAR
gatifloxacin solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |
Revised: 4/2018
Document Id: 8d03c614-14ad-4602-a2b6-e27abbdd90f1
Set id: a5b0725e-9b7e-426b-ab1c-fcca3b1972fd
Version: 14
Effective Time: 20180401
Allergan, Inc.