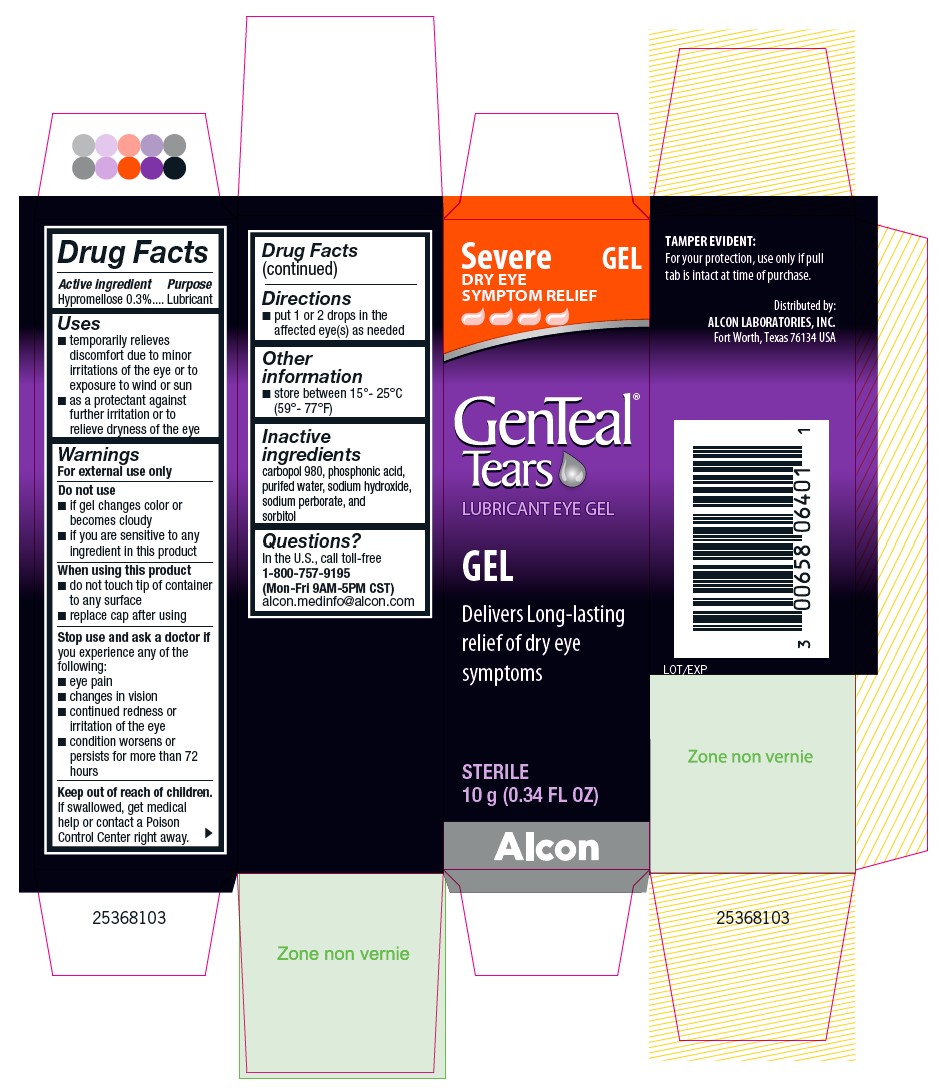
Uses
- temporarily relieves discomfort due to minor irritations of the eye or to exposure to wind or sun
- as a protectant against further irritation or to relieve dryness of the eye
Do not use
- if gel changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
Stop use and ask a doctor if you experience any of the following:
- eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients
carbopol 980, phosphonic acid, purified water, sodium hydroxide, sodium perborate, and sorbitol
PRINCIPAL DISPLAY PANEL
Severe DRY EYE SYMPTOM RELIEF
GEL
GenTeal® Tears
LUBRICANT EYE GEL
GEL
Delivers Long-lasting relief of dry eye symptoms
STERILE
10 g (0.34 FL OZ)
TAMPER EVIDENT:
For your protection, use only if pull tab is intact at time of purchase.
Distributed by:
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Lot/Exp
Alcon
25368103
Severe DRY EYE SYMPTOM RELIEF
GenTeal® Tears
LUBRICANT EYE GEL
GEL
Delivers long-lasting relief of dry eye symptoms
STERILE 10g (0.34 FL OZ)
Active Ingredient: Hypromellose (0.3%) Uses: See carton for Uses.
Warnings: See carton for Warnings. When using this product do not touch tip of container to any surface. Replace cap after using. Directions: Put 1 or 2 drops in the affected eye(s) as needed. Other Information: Store between 15°-25°C (59°-77°F).
TAMPER EVIDENT: For your protection, use only if pull tab is intact at the time of purchase.
Distributed by:
ALCON LABORATORIES, INC.
6201 South Freeway, Fort Worth, Texas 76134 USA
ALCON
16027802
