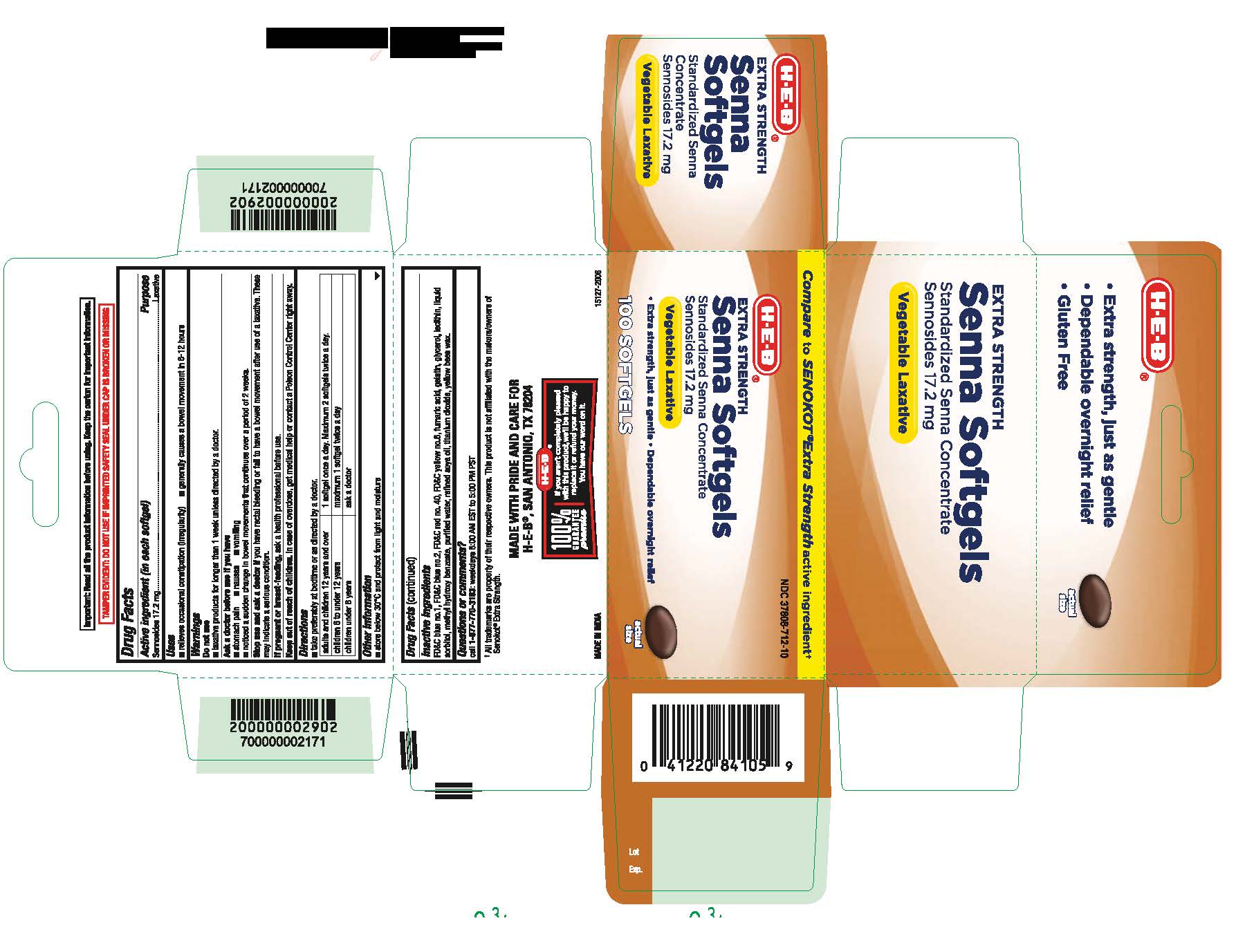
Uses
■ relieves occasional constipation (irregularity)
■ generally produces a bowel movement within 6-12 hours
Ask a doctor before use if you have
■ stomach pain
■ nausea
■ vomiting
■ noticed a sudden change in bowel habits that continues over a period of 2 weeks
Stop use and consult a doctor if
you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away
Directions
■ take preferably at bedtime or as directed by a doctor.
adults and children 12 years and over
■ 1 capsule once a day. Maximum 2 capsules twice a day.
children 6 to under 12 years
■ maximum 1 capsule twice a day
children under 6 years
■ ask a doctor