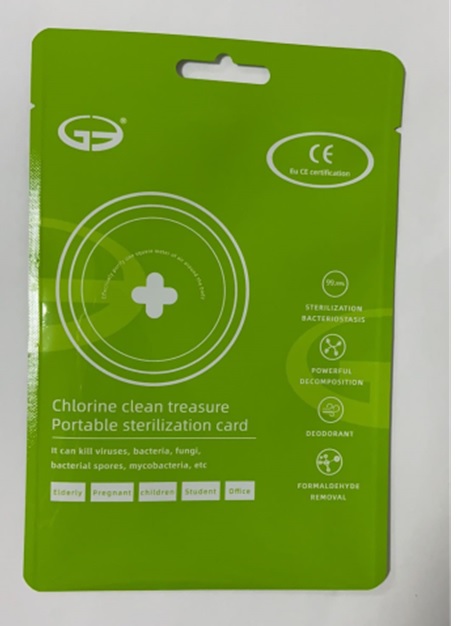
CLO2--Portable sterilization card
Active Ingredient(s)
Chlorine dioxide 10g/15g. Purpose: Antiseptic
Purpose
Antiseptic, Portable sterilization card
Use
Portable sterilization card to help reduce bacteria that potentially can cause disease.
Warnings
For external use only. Flammable. Keep away from heat or flame
Do not use
- in children less than 2 months of age
- on open skin wounds
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Take it with you, the gas it releases can kill bacteria
- Supervise children under 6 years of age when using this product to avoid swallowing.
Other information
- Store between 15-30C (59-86F)
- Avoid freezing and excessive heat above 40C (104F)
Inactive ingredients
Urea nitrate
Magnesium sulfate
Calcium sulphate
Superabsorbent acrylic resin
Beta cyclodextrins
Package Label - Principal Display Panel
Shenzhen Haiyin Hongye Technology Co., Ltd.
