KEKE HAND SANITIZER 500ML 01- alcohol liquid
Shenzhen Lantern Scicence Co.,Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
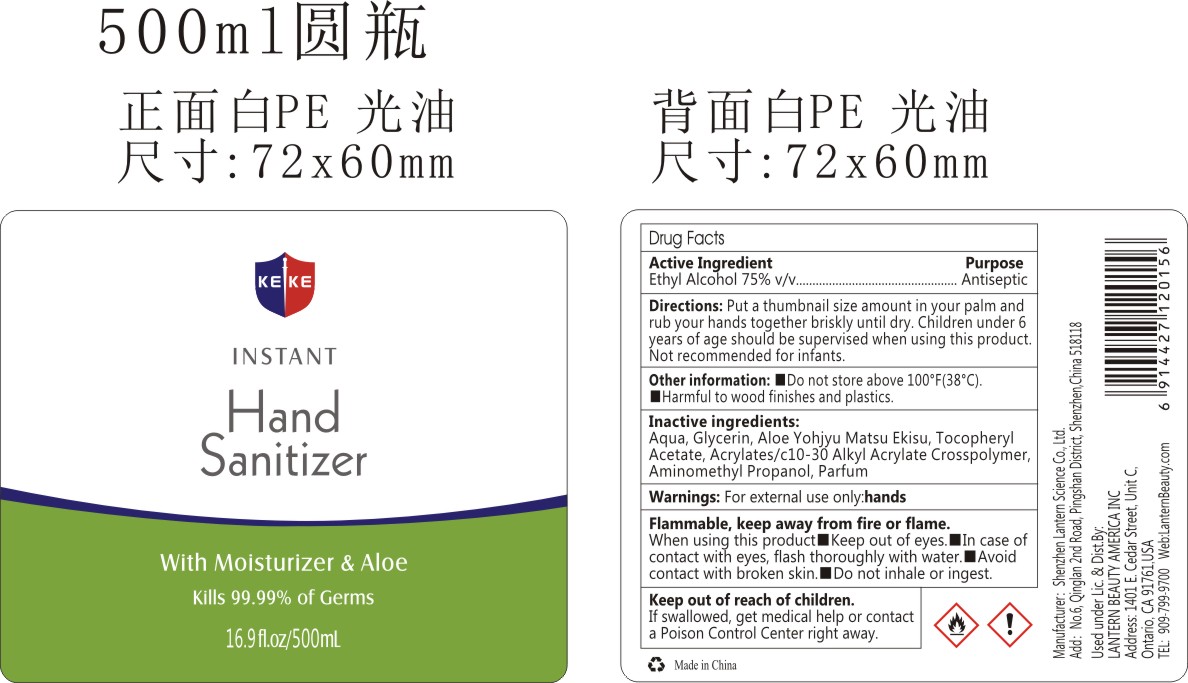
Active Ingredient
Active Ingredient Purpose
Ethyl Alcohol 75%(V/V) Antiseptic
keep out of reach of children
If swallowed,get medical help or contact a Poison Control Center right away.
Recommended for repeated use.
use anywhere without water.
Warning
For external use only:hands
Flammable,keep away from fire or flame.
For external use only.
Flammable, keep away from heat and flame.
Discontinue if skin becomes irritated and ask a doctor .
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a poson control center immediately.
Inactive ingredients
Aqua,Glycerin,Aloe Yohjyu Matsu Ekisu,Tocopheryl Acetate,Acrylates/C10-30 Alkyl Acrylate Crosspolymer,Aminomethyl Propanol,Parfum.
Directions
put a thumbnail size amount in your palm and rub your hands together briskly until dry.Children under 6 years of age should be supervised when using this product.Not recommended for infants.
When using this product
keep out of eyes. In case of contact with eyes, flush thoroughly with water.
Do not inhale or ingest.
Avoid contact with broken skin.
Other information
Do not store above 105F.
May discolor some fabrics.
Harmful to wood finishes and plastics.
Other information
Do not store above 100℉(38℃)
Harmful to wood finishes and plastics.
Shenzhen Lantern Scicence Co.,Ltd.
