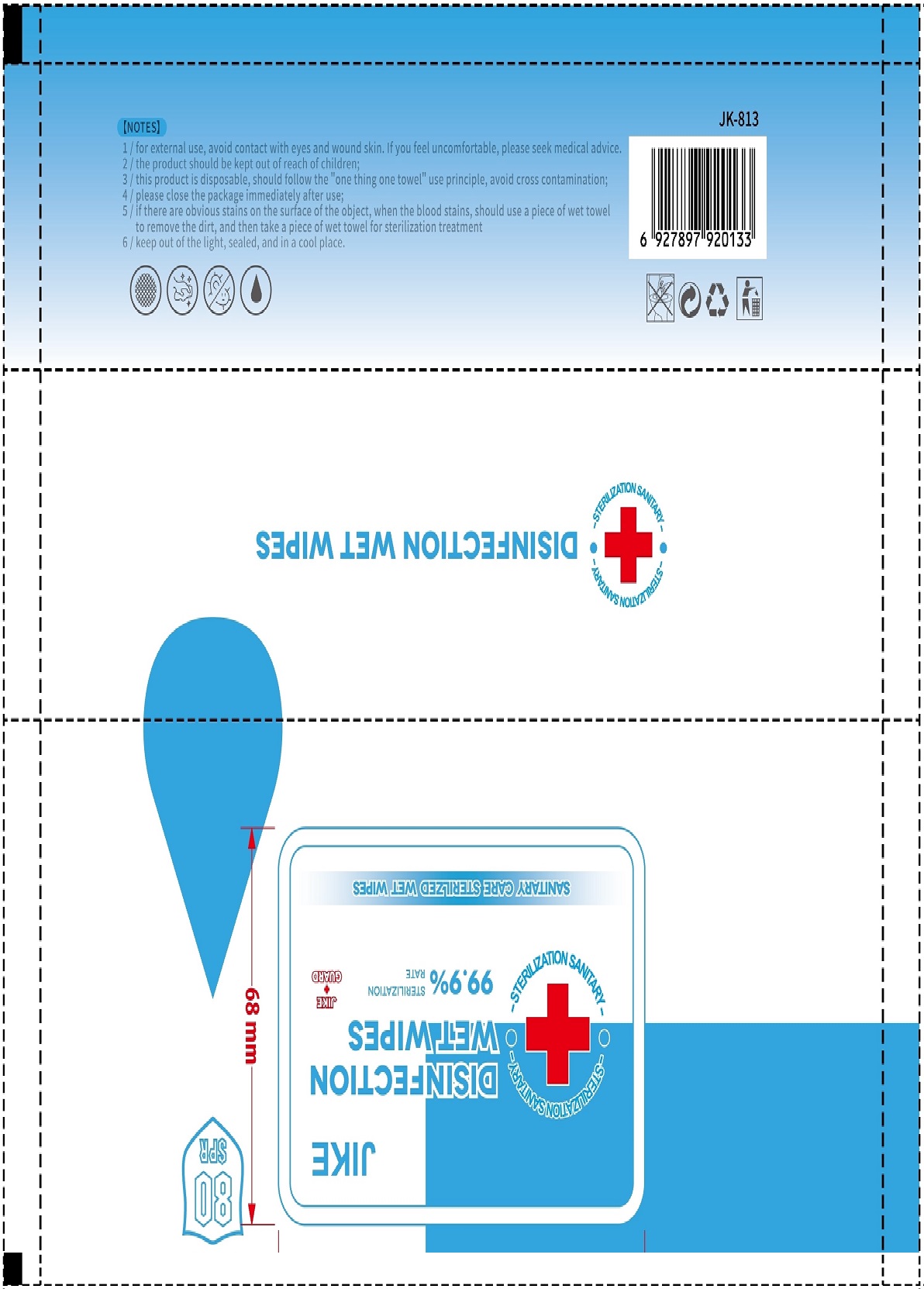
DISINFECTION WET WIPES- disinfection wet wipes patch
Zhejiang Jiayan Daily Commodity Co., Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Phenoxyethanol, 0.5% w/w;
Benzalkonium Chloride, 0.15% w/w.
Inactive ingredient
Ethylhexylglycerin; Didecyldimonium Chloride; Propylene Glycol; Glycerin; Water.
Purpose
Kill the intestinal pathogenic bacterium, staphylococcus aureus pseudomonas aeruginosa candida albicans.
When using
Please close the pakage immediately after use.
If there are obvious stains on the surface of the object, when the blood stains, should use a piece of wet towel to remove the dirty, and then take a piece of wet towel for sterilization treatment.
Do not use
For external use, avoid contact with eyes and wound skin.
Stop use
If you feel uncomfortable, please seek medical advice.
Keep out of reach of children
The product should be kept out of reach of children.
Indications & usage
Applicable to the hand skin, object surface and medical equipment surface cleaning sterilization.
Dosage & administration
Open the package of wet towel and take out and unfold the wet towel.
From one side of the surface of the object, from the top down by the s-type wipe to the entire surface, action for 3 minutes.
Warnings
For external use, avoid contact with eyes and wound skin.
This product is disposable, should follow the "one thing one towel" use principle, avoid cross contamination.
Other information
Keep out of the light, sealed, and in a cool place.
Package label. Principal display panel