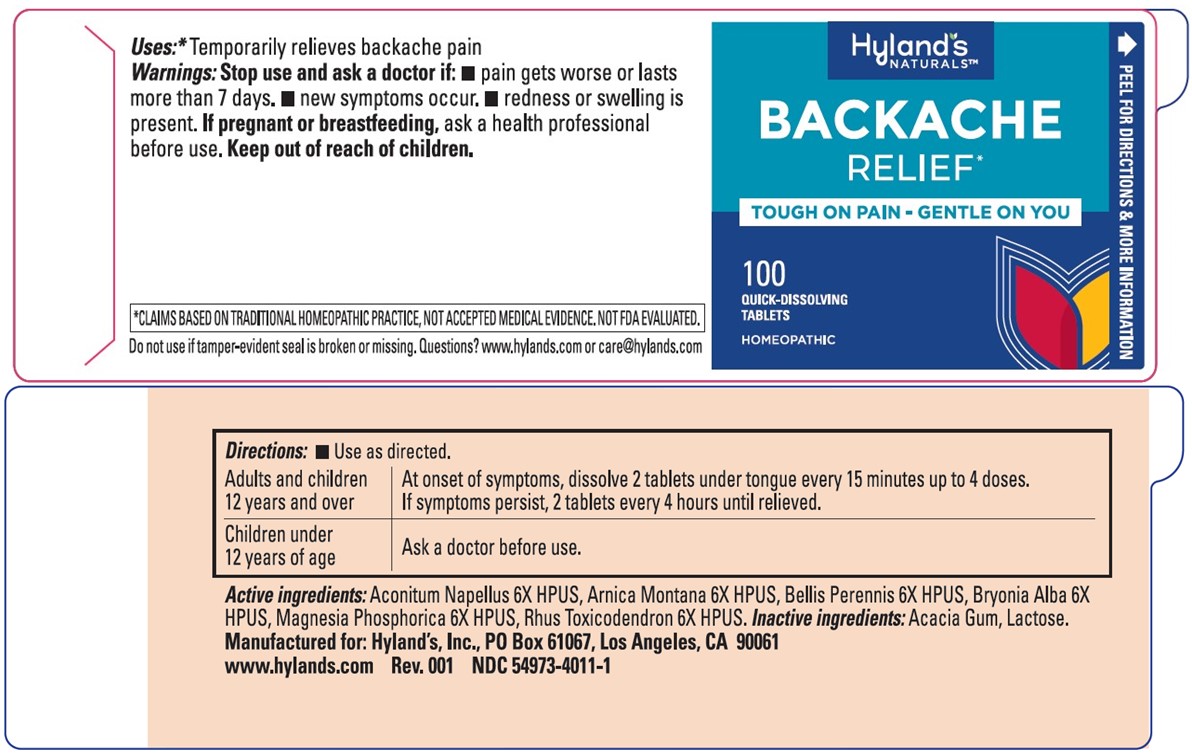
BACKACHE RELIEF- aconitum napellus, bryonia alba root, magnesium phosphate, dibasic trihydrate, bellis perennis, toxicodendron pubescens leaf and arnica montana tablet
Hyland's Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts
Active ingredients
|
Active ingredients
|
Purpose
|
|
Aconitum Napellus 6X HPUS
|
backache pain
|
|
Arnica Montana 6X HPUS
|
backache pain
|
|
Bellis Perennis 6X HPUS
| backache pain |
|
Bryonia Alba 6X HPUS
| backache pain |
|
Magnesia Phosphorica 6X HPUS
| backache pain |
|
Rhus Toxicodendron 6X HPUS
| backache pain |
"HPUS" indicates that the active ingredients are in the official Homeopathic
Pharmacopoeia of the United States.
Uses
■ Temporarily relieves backache pain
Warnings
Stop use and ask a doctor if
■ pain gets worse or lasts more than 7 days.
■ new symptoms occur.
■ redness or swelling is present
If pregnant or breastfeeding
Ask a health professional before use.
Keep out of reach of children.
Directions
■ Use as directed.
Adults and children
12 years and over
|
At onset of symptoms, dissolve 2 tablets under tongue every
15 minutes up to 4 doses. If symptoms persist, 2 tablets every
4 hours until relieved.
|
Children under
12 years of age
| Ask a doctor before use. |
Inactive ingredients
Acacia Gum, Lactose.
Questions?
www.hylands.com or care@hylands.com
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
DO NOT USE IF TAMPER-EVIDENT SEAL IS BROKEN OR MISSING.
Principal Display Panel
NDC 54973-4011-1
100 TABLETS

Hyland's Inc.

