AHFS Category: 36:84
Rx only
Diagnostic Antigen
(Aid in the detection of infection with Mycobacterium tuberculosis)
FOR INTRADERMAL USE
Polysorbate 80 Stabilized Solution of Tuberculin Purified Protein Derivative for Tuberculin Testing in Humans
DESCRIPTION
TUBERSOL® Tuberculin Purified Protein Derivative (Mantoux) (PPD) (1) for intradermal tuberculin testing is prepared from a large Master Batch Connaught Tuberculin (CT68) (2) and is a cell-free purified protein fraction obtained from a human strain of Mycobacterium tuberculosis grown on a protein-free synthetic medium and inactivated. (2) The use of a standard preparation derived from a single batch (CT68) has been adopted in order to eliminate batch to batch variation by the same manufacturer. (2)
TUBERSOL is a clear, colorless liquid.
| TUBERSOL contains: | |
| Purified protein derivative of M. tuberculosis | 5 TU per 0.1 mL |
| Polysorbate 80 | 0.0006% |
| Phenol | 0.22% to 0.35% w/v |
| in sterile isotonic phosphate buffered saline. | |
Before release, each successive lot is tested for potency in comparison with a reference standard.
Independent studies conducted by the US Public Health Service in humans have determined the amount of CT68 in stabilized solution necessary (3)(4)(5) to produce bio-equivalency with Tuberculin PPD-S (in phosphate buffer without polysorbate 80) using 5 US units (TU) Tuberculin PPD-S as the standard.
CLINICAL PHARMACOLOGY
Mechanism of Action
The sensitization following infection with mycobacteria occurs primarily in the regional lymph nodes. Small lymphocytes (T lymphocytes) proliferate in response to the antigenic stimulus to give rise to specifically sensitized lymphocytes. After 3-8 weeks, these lymphocytes enter the blood stream and circulate for years. (6) Subsequent restimulation of these sensitized lymphocytes with the same or a similar antigen, such as the intradermal injection of TUBERSOL, evokes a local reaction mediated by these cells. (7)
Characteristically, delayed hypersensitivity reactions to tuberculin begin at 5 to 6 hours, are maximal at 48 to 72 hours and subside over a period of days. The resultant immune response consists of induration due to cell infiltration and occasionally vesiculation and necrosis. Clinically, a delayed hypersensitivity reaction to tuberculin is a manifestation of previous infection with M tuberculosis or a variety of non-tuberculosis bacteria. In most cases sensitization is induced by natural mycobacterial infection or by vaccination with BCG Vaccine.
INDICATIONS AND USAGE
TUBERSOL Tuberculin Purified Protein Derivative (Mantoux), is indicated to aid diagnosis of tuberculosis infection (TB) in persons at increased risk of developing active disease.
The Centers for Disease Control and Prevention (CDC) have published guidelines regarding populations that would benefit from tuberculin skin testing (TST). Current recommendations can be accessed at: http://www.cdc.gov/tb/publications/factsheets/testing.htm.
Previous BCG vaccination is not a contraindication to tuberculin testing. The skin-test results of BCG vaccinated persons can be used to support or exclude the diagnosis of TB infection. However, an FDA-approved interferon gamma release assay is preferred over tuberculin skin test for persons 5 years of age and older who were previously vaccinated with BCG. (8)
CONTRAINDICATIONS
Allergy to any component of TUBERSOL or an anaphylactic or other allergic reaction to a previous test of tuberculin PPD is a contraindication to the use of TUBERSOL. (See DESCRIPTION and HOW SUPPLIED.)
TUBERSOL should not be administered to:
- Persons who have had a severe reaction (e.g., necrosis, blistering, anaphylactic shock, or ulcerations) to a previous TST,
- Persons with documented active tuberculosis or a clear history of treatment for TB infection or disease, (9)
- Persons with extensive burns or eczema.
WARNINGS
Hypersensitivity
Allergic reactions may occur following the use of TUBERSOL even in persons with no prior history of hypersensitivity to the product components. (10) Epinephrine injection (1:1,000) and other appropriate agents used for the control of immediate allergic reactions must be immediately available.
PRECAUTIONS
General
Diagnostic Limitations
False positive or false negative tuberculin skin test reactions may occur in some individuals. (See Interpretation of the Test.)
False positive tuberculin reaction tests occur in individuals who have been infected with other mycobacteria, including vaccination with BCG.
Not all infected persons will have a delayed hypersensitivity reaction to a tuberculin test.
Many factors have been reported to cause a decreased ability to respond to the tuberculin test in the presence of tuberculous infection. (See Interpretation of the Test.)
Information for Patients
Prior to administration of TUBERSOL, the patient's current health status and medical history should be reviewed. The physician should review the patient's immunization history for possible sensitivity to components of TUBERSOL.
The healthcare provider should inform the patient of the need to return for the reading of the test. Self-reading of the test has been shown to be inaccurate and unreliable.
The healthcare provider should give the patient a permanent personal record. In addition, it is essential that the health professional record the testing history in the permanent medical record of each patient. This permanent office record should contain the name of the product, date given, dose, manufacturer, and lot number, as well as the test result in millimeters of induration (including 0 mm, if appropriate). Reporting results only as negative or positive is not satisfactory.
Drug Interactions
Reactivity to the test may be depressed or suppressed in persons who are receiving corticosteroids or immunosuppressive agents. (7)
Reactivity to TUBERSOL may be temporarily depressed by certain live virus vaccines (measles, mumps, rubella, oral polio, yellow fever, and varicella). If a parenteral live attenuated virus vaccine has been administered recently, tuberculin testing should be delayed for >1 month after vaccination. (7)(11) (See Interpretation of the Test.)
When tuberculin screening is required at the same time as a measles-containing vaccine or other parenteral live attenuated virus vaccine, simultaneous administration of TUBERSOL and the vaccine at separate sites is the preferred option.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
TUBERSOL has not been evaluated for its carcinogenic or mutagenic potentials or impairment of fertility.
Pregnancy
Animal reproduction studies have not been conducted with TUBERSOL. It is also not known whether TUBERSOL can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. TUBERSOL should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether TUBERSOL is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when TUBERSOL is administered to a nursing woman.
Pediatric Use
There is no contraindication to tuberculin skin testing of infants. Infants <6 months of age who are infected with M. tuberculosis may not react to TUBERSOL. (See Interpretation of the Test.)
ADVERSE REACTIONS
Induration at the TUBERSOL injection site is the expected reaction for a positive skin test. (See Interpretation of the Test.)
The information pertaining to adverse events has been compiled from historical clinical studies and post-marketing experience with TUBERSOL.
General disorders and administration site conditions
- Injection site pain, injection site pruritus and injection site discomfort.
- Injection site erythema or injection site rash (without induration) occurring within 12 hours of testing. These reactions do not indicate TB infection.
- Injection site hemorrhage and injection site hematoma up to three days after the administration of the test.
- Injection site vesicles, injection site ulcer or injection site necrosis in highly sensitive persons.
- Injection site scar as a result of strongly positive reactions.
- Pyrexia
Immune system disorders
- Hypersensitivity, including anaphylaxis/anaphylactic reactions, angioedema, urticaria
Nervous system disorders
- Presyncope, syncope (including syncope associated with tonic-clonic movements and other seizure-like activity) sometimes resulting in transient loss of consciousness with injury
Reporting of Adverse Events
To report SUSPECTED ADVERSE REACTIONS, contact the Pharmacovigilance Department, Sanofi Pasteur Inc., Discovery Drive, Swiftwater, PA 18370 or call 1-800-822-2463 (1-800-VACCINE) or Food and Drug Administration (FDA) MEDWATCH Program at 1-800-332-1088 and www.fda.gov/medwatch.
DOSAGE AND ADMINISTRATION
Dosage
Five (5) tuberculin units (TU) per test dose of 0.1 mL is the standard strength used for intradermal (Mantoux) testing.
Method of Administration
TUBERSOL is indicated for intradermal injection only. Do not inject intravenously, intramuscularly, or subcutaneously. If subcutaneous injection occurs, the test cannot be interpreted.
Inspect for extraneous particulate matter and/or discoloration before use. If these conditions exist, do not administer the product.
Use a separate syringe and needle for each injection. (12)
The following procedure is recommended for performing the Mantoux test:
- The preferred site of the test is the volar aspect of the forearm. Avoid areas on the skin that are red or swollen. Avoid visible veins.
- Clean the skin site with a suitable germicide and allow the site to dry prior to injection of the antigen.
- Administer the test dose (0.1 mL) of TUBERSOL with a 1 mL syringe calibrated in tenths and fitted with a short, one-quarter to one-half inch, 26 or 27 gauge needle.
- Wipe the stopper of the vial with a suitable germicide and allow to dry before needle insertion. Then insert the needle gently through the stopper and draw 0.1 mL of TUBERSOL into the syringe. Avoid injection of excess air with removal of each dose so as not to over pressurize the vial and possibly cause seepage at the puncture site.
- Insert the point of the needle into the most superficial layers of the skin with the needle bevel pointing upward and administer the dose by slow intradermal injection. If the intradermal injection is performed properly, a definite pale bleb will rise at the needle point, about 10 mm (3/8") in diameter. This bleb will disperse within minutes. Do not dress the site.
- A drop of blood may appear at the administration site following injection. Blot the site lightly to remove the blood but avoid squeezing out the injected tuberculin test fluid.
In the event of an improperly performed injection (i.e., no bleb formed), repeat the test immediately at another site, at least 2 inches from the first site and circle the second injection site as an indication that this is the site to be read.
Inform the patient of the need to return for the reading of the test by a trained health professional. Self-reading may be inaccurate and is strongly discouraged.
Interpretation of the Test
The skin test should be read by a trained health professional 48 to 72 hours after administration of TUBERSOL. Skin test sensitivity is indicated by induration only; redness should not be measured.
Measure the diameter of induration transversely to the long axis of the forearm and record the measurement in millimeters (including 0 mm). (7) The tip of a ballpoint pen, gently pushed at a 45° angle toward the site of injection, will stop at the edge of induration.
Also record presence and size (if present) of necrosis and edema, although these are not used in the interpretation of the test.
Positive Reactions
Tuberculin reactivity may indicate latent infection, prior infection and/or disease with M. tuberculosis and does not necessarily indicate the presence of active tuberculous disease. Persons showing positive tuberculin reactions should be considered positive by current public health guidelines and referred for further medical evaluation. (7)(9) The repeated testing of uninfected persons does not sensitize them to TUBERSOL. (6)(7)(9)
The significance of induration measurements in diagnosing latent TB infection must be considered in terms of the patient's history and the risk of developing active TB disease as indicated in Table 1. (9)
| Reaction ≥5 mm of Induration | Reaction ≥10 mm of Induration | Reaction ≥15 mm of Induration |
|---|---|---|
| HIV-positive persons Recent contacts of tuberculosis (TB) case patients Fibrotic changes on chest radiograph consistent with prior TB Patients with organ transplants and other immunosuppressed patients (receiving the equivalent of ≥15 mg/d of prednisone for 1 month or more)* | Recent immigrants (i.e., within the last 5 yrs) from high prevalence countries Injection drug users Residents or employees† of the following high-risk congregate settings: prisons and jails, nursing homes and other long-term facilities for the elderly, hospitals and other healthcare facilities, residential facilities for patients with acquired immunodeficiency syndrome (AIDS) and homeless shelters Mycobacteriology laboratory personnel Persons with the following clinical conditions that place them at high risk: silicosis, diabetes mellitus, chronic renal failure, some hematologic disorders (e.g., leukemias and lymphomas), other specific malignancies (e.g., carcinoma of the head or neck and lung), weight loss of ≥10% of ideal body weight, gastrectomy and jejunoileal bypass Children younger than 4 yrs of age or infants, children, and adolescents exposed to adults at high-risk | Persons with no risk factors for TB |
A TST conversion is defined as an increase of ≥10 mm of induration within a 2-year period, regardless of age. (9)
The possibility should be considered that the skin test sensitivity may also be due to a previous contact with atypical mycobacteria or previous BCG vaccination. (7)(9)
Negative Reactions
An individual who does not show a positive reaction to 5 TU on the first test, but is suspected of being TB positive, may be retested with 5 TU. (See Booster Effect and Two-Step Testing.) Any individual who does not show a positive reaction to an initial injection of 5 TU, or a second test with 5 TU may be considered as tuberculin negative.
False Positive Reactions
False positive tuberculin reactions can occur in individuals who have been infected with other mycobacteria, including vaccination with BCG. (7) However, a diagnosis of M. tuberculosis infection and the use of preventive therapy should be considered for any BCG-vaccinated person who has a positive TST reaction, especially if the person has been, or is, at increased risk of acquiring TB infection. (See INDICATIONS AND USAGE.) (13)(14)
False Negative Reactions
Not all infected persons will have a delayed hypersensitivity reaction to a tuberculin test.
In those who are elderly or those who are being tested for the first time, reactions may develop slowly and may not peak until after 72 hours.
Since tuberculin sensitivity may take up to 8 weeks to develop following exposure to M. tuberculosis (see Mechanism of Action), persons who have a negative tuberculin test <8 weeks following possible TB exposure should be retested ≥8-10 weeks following the last known or suspected exposure. (15)
Altered Immune Status
Impaired or attenuated cell mediated immunity (CMI) can potentially cause a false negative tuberculin reaction. Many factors have been reported to cause a decreased ability to respond to the tuberculin test in the presence of tuberculous infection including viral infections (e.g., measles, mumps, chickenpox and HIV), live virus vaccinations (e.g., measles, mumps, rubella, oral polio and yellow fever), overwhelming tuberculosis, other bacterial infections, leukemia, sarcoidosis, fungal infections, metabolic derangements, low protein states, diseases affecting lymphoid organs, drugs (corticosteroids and many other immunosuppressive agents), and malignancy or stress. (7)(16)(17) A TST should be deferred for patients with major viral infections or live-virus vaccination in the past month. Persons with the common cold may be tuberculin tested.
Because TST results in HIV-infected individuals are less reliable as CD4 counts decline, screening should be completed as early as possible after HIV-infection occurs. (17)
Booster Effect and Two-Step Testing
If tuberculin testing will be conducted at regular intervals, for instance among healthcare workers or prison workers, two-step testing should be performed as a baseline to avoid interpreting a booster effect as a tuberculin conversion. If the first test showed either no reaction or a small reaction, the second test should be performed one to four weeks later. Both tests should be read and recorded at 48 to 72 hours. Patients with a second tuberculin test (booster) response of ≥10 mm should be considered to have experienced past TB infection. (13)(18)
Persons who do not boost when given repeat tests at one week, but whose tuberculin reactions change to positive after one year, should be considered to have newly acquired tuberculosis infection and managed accordingly. (6)
HOW SUPPLIED
TUBERSOL Tuberculin Purified Protein Derivative (Mantoux), bioequivalent to 5 US units (TU) PPD-S per test dose (0.1 mL) is supplied in:
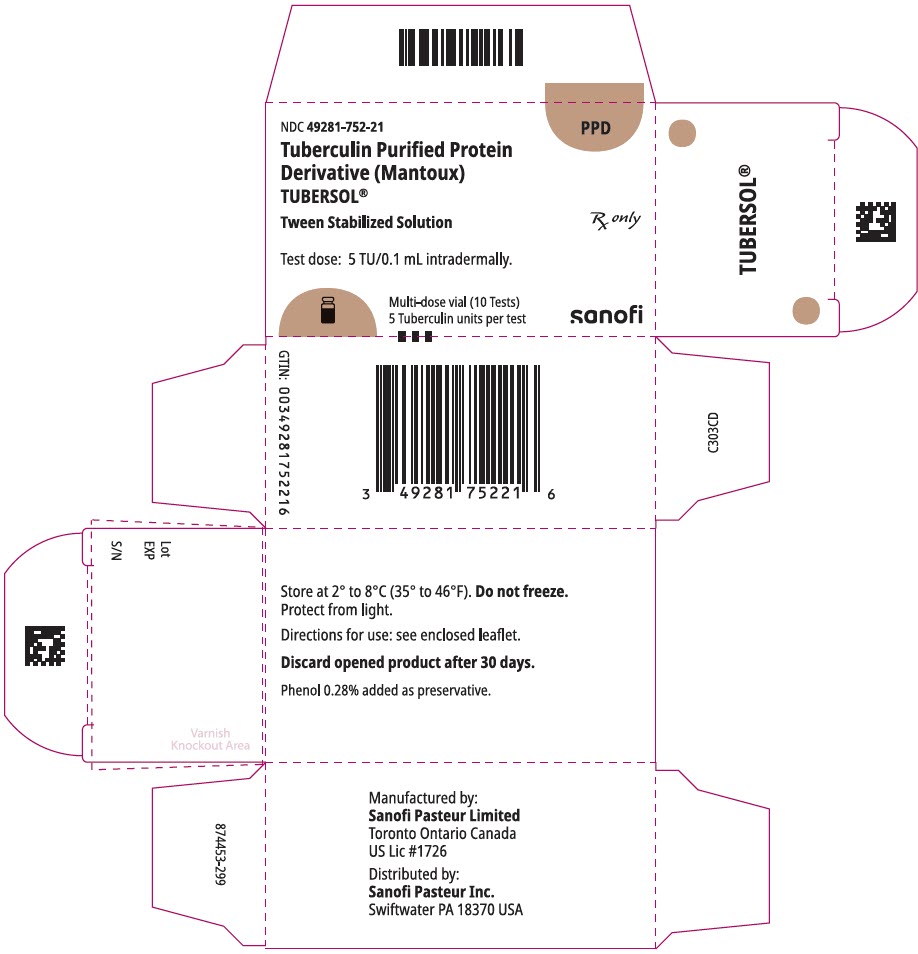
1 mL multi-dose vial (10 tests). NDC No. 49281-752-78; package of 1 vial, NDC No. 49281-752-21
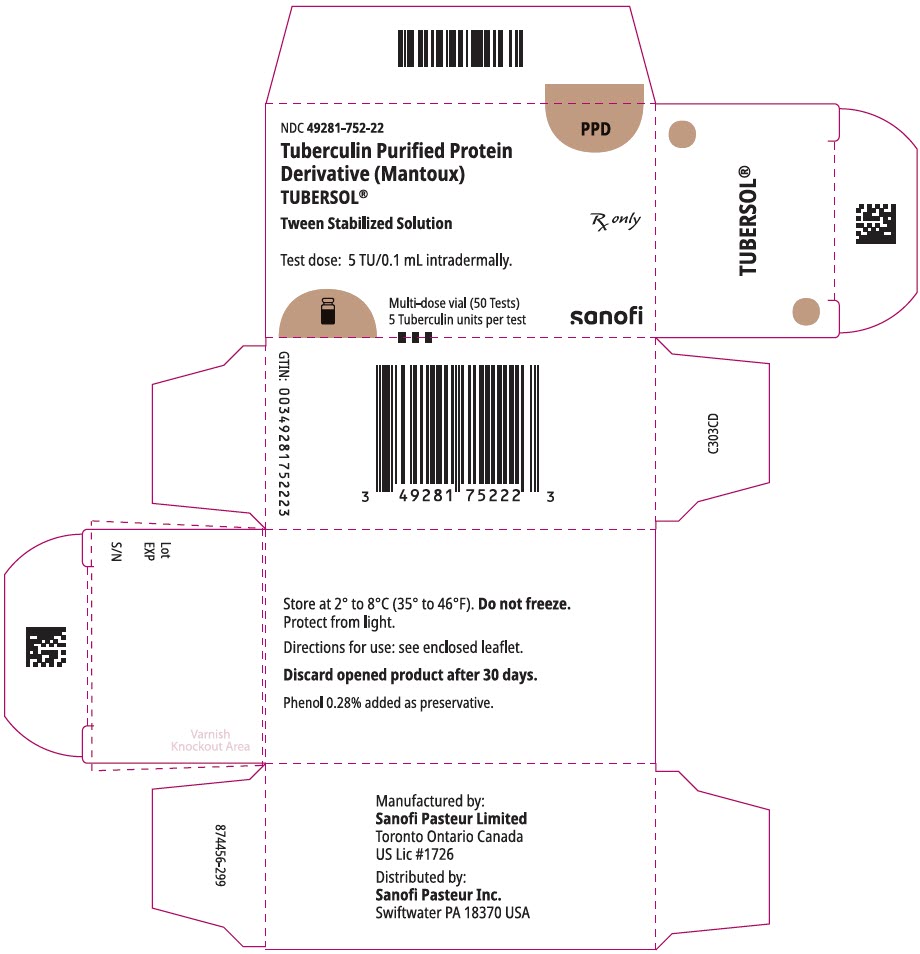
5 mL multi-dose vial (50 tests). NDC No. 49281-752-98; package of 1 vial, NDC No. 49281-752-22
The stopper of the vial for this product does not contain natural latex rubber.
STORAGE
Store at 2° to 8°C (35° to 46°F). (19) Do not freeze. Discard product if exposed to freezing.
Protect from light. Tuberculin PPD solutions can be adversely affected by exposure to light. The product should be stored in the dark except when doses are actually being withdrawn from the vial. (20)
A vial of TUBERSOL which has been entered and in use for 30 days should be discarded. (21)
Do not use after expiration date.
REFERENCES
- 1
- Landi S. Preparation, purification, and stability of tuberculin. Appl Microbiol 1963;11:408-412.
- 2
- Landi S, et al. Preparation and characterization of a large batch of tuberculin purified protein derivative (PPD-CT68). Ann Scalvo.1980;22:889-907.
- 3
- Landi S, et al. Adsorption of tuberculin PPD to glass and plastic surfaces. Bull. WHO 1966;35:593-602.
- 4
- Landi S, et al. Disparity of potency between stabilized and nonstabilized dilute tuberculin solutions. Am Rev Respir Dis 1971;104:385-393.
- 5
- Landi S, et al. Stability of dilute solutions of tuberculin purified protein derivative. Tubercle 1978;59:121-133.
- 6
- Menzies D. Interpretation of repeated tuberculin tests. Am J Respir Crit Care Med 1999;159:15-21.
- 7
- American Thoracic Society: Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-1395.
- 8
- CDC. Updated Guidelines for Using Interferon Gamma Release Assays to Detect Mycobacterium tuberculosis Infection - United States, 2010. MMWR 2010; 59 (RR-5):1-25.
- 9
- CDC. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR 2000;49(RR-6):23-5.
- 10
- Froeschle JE, et al. Immediate hypersensitivity reactions after use of tuberculin skin testing. Clin Infect Dis 2002;34:e12-13.
- 11
- Brickman HF, et al. The timing of tuberculin tests in relation to immunization with live viral vaccines. Pediatrics: 1975;55:392-396.
- 12
- CDC. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). MMWR 2002;51(RR-2):1-36.
- 13
- CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR 2005;54(RR-17):1-141.
- 14
- CDC. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR 1996; 45(RR-4):1-18.
- 15
- CDC. Guidelines for the Investigation of Contacts of Persons with Infectious Tuberculosis: Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR 2005;54(RR-15):1-37.
- 16
- Mori and Shiozawa. Suppression of tuberculin hypersensitivity caused by rubella infection. Am Rev Respir Dis 1985;886-888.
- 17
- CDC. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Recommendations from the CDC, the National Institutes of Health, and the HIV Medicine Association of Infectious Diseases Society of America. MMWR 2009;58(RR-4):1-207.
- 18
- CDC. Prevention and control of tuberculosis in correctional and detention facilities: Recommendations from the CDC. MMWR 2006;55(RR-9):1-44.
- 19
- Landi S, et al. Stability of dilute solution of tuberculin purified protein derivative at extreme temperatures. J Biol Stand 1981;9:195-199.
- 20
- Landi S, et al. Effect of light on tuberculin purified protein derivative solutions. Am Rev Respir Dis 1975;111:52-61.
- 21
- Landi S, et al. Effect of oxidation on the stability of tuberculin purified protein derivative (PPD) In: International Symposium on Tuberculins and BCG Vaccine. Basel: International Association of Biological Standardization, 1983. Dev Biol Stand 1986;58:545-552.
Manufactured by:
Sanofi Pasteur Limited
Toronto Ontario Canada
Distributed by:
Sanofi Pasteur Inc.
Swiftwater PA 18370 USA
Product Information as of
October 2021
TUBERSOL is a registered trademark of Sanofi Pasteur. ©, 2021 TUBERSOL®, Sanofi Pasteur Limited - All rights reserved
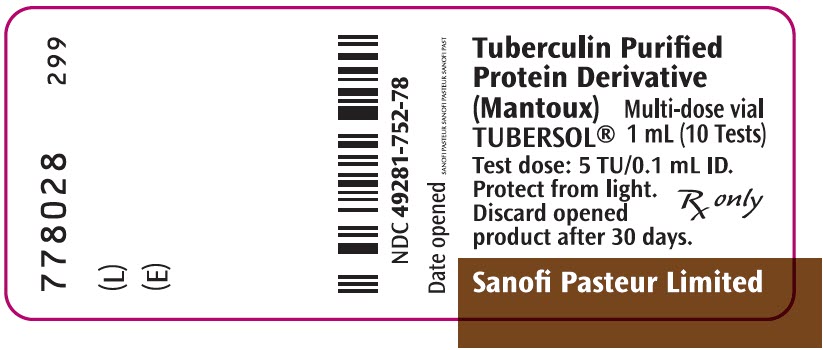
PRINCIPAL DISPLAY PANEL - 1 mL Vial Label
Tuberculin Purified
Protein Derivative
(Mantoux)
TUBERSOL®
Multi-dose vial
1 mL (10 Tests)
Test dose: 5 TU/0.1 mL ID.
Protect from light.
Discard opened
product after 30 days.
Rx only
Sanofi Pasteur Limited
PRINCIPAL DISPLAY PANEL - 1 mL Vial Carton
NDC 49281-752-21
PPD
Tuberculin Purified Protein
Derivative (Mantoux)
TUBERSOL®
Tween Stabilized Solution
Test dose: 5 TU/0.1 mL intradermally.
Multi-dose vial (10 Tests)
5 Tuberculin units per test
Rx only
sanofi