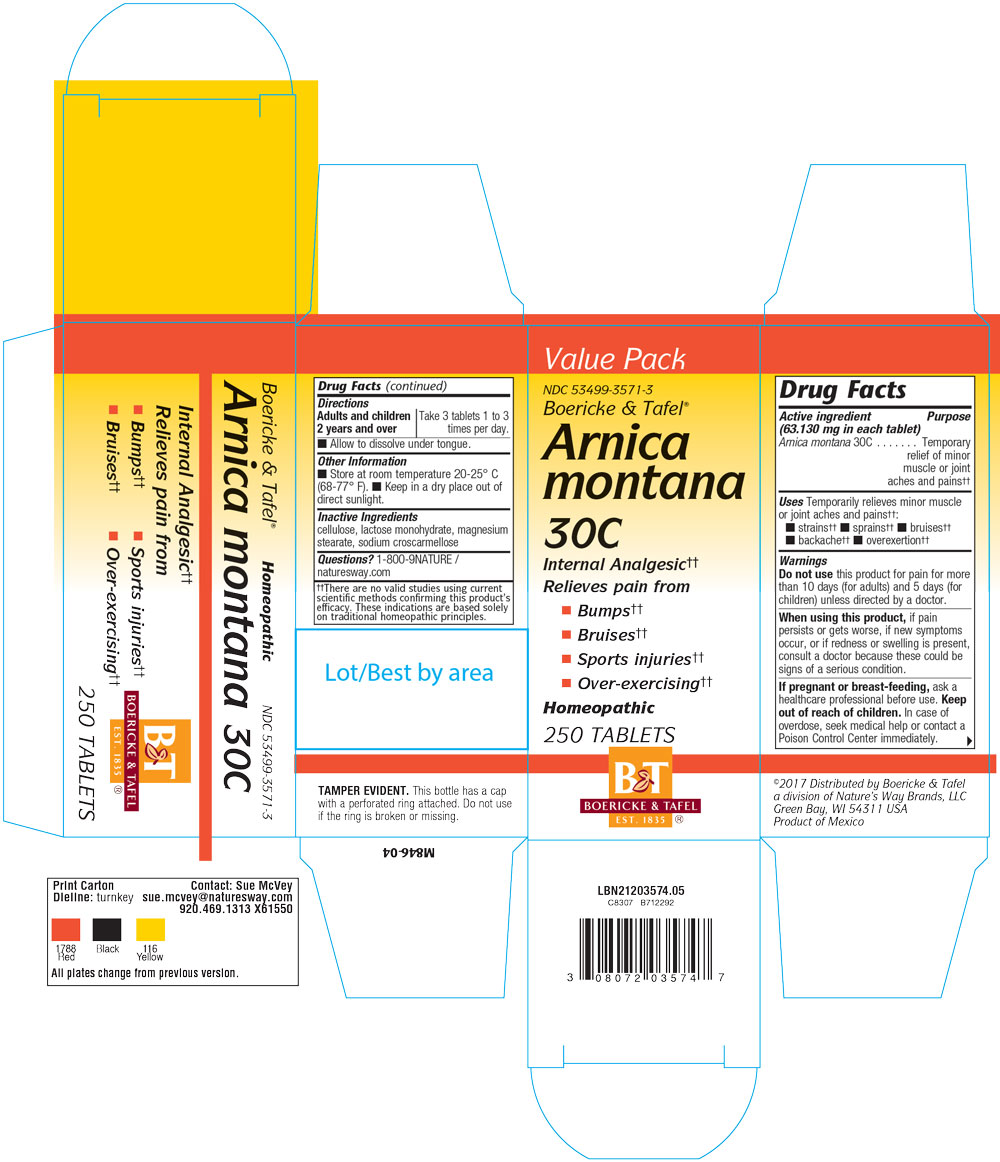
ARNICA MONTANA 30C- arnica montana tablet
Schwabe Mexico, S.A. de C.V.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredient
Arnica Montana 30C
Inactive Ingredients
CELLULOSE
CROSCARMELLOSE SODIUM
LACTOSE MONOHYDRATE
MAGNESIUM STEARATE
Dosage & Administration
Directions: Adults or children over 2 years: Take 3 tablets 1-3 times a day.
Allow to dissolve under tongue.
Indications & Usage
Temporarily relieves minor muscle or joint aches and pains from strains, sprains, bruises, backache, over extertion.
Purpose
For the temporary relief of minor muscle or joint aches and pains from strains, sprains, bruises, backache and over exertion.
Warnings
Do not use this product for pain for more than 10 days (adult) or 5 days (children) unless directed by a doctor.
When using
When using this product, if pain persists or gets worse, if new symptoms occur, or if redness or swelling is present, consult a doctor because these could be signs of a serious condition.
Keep Out of Reach of Children
Keep out of reach of children.
Overdosage
In case of overdose, seek medical help or contact a Poison Control Center immediately.
Schwabe Mexico, S.A. de C.V.