OLIKA HAND SANITIZER ORANGE BLOSSOM- ethyl alcohol liquid
OLIKA HAND SANITIZER LAVENDER- ethyl alcohol liquid
OLIKA HAND SANITIZER CHARCOAL- ethyl alcohol liquid
OLIKA HAND SANITIZER CUCUMBER BASIL- ethyl alcohol liquid
OLIKA HAND SANITIZER MINT CITRUS- ethyl alcohol liquid
Olika Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
These products are hand sanitizers manufactured according to the 1994 tentative final monograph for antiseptics with additional guidance taken from subsequent rule makings and guidances.
The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation).
- Alcohol (ethanol) (USP or Food Chemical Codex (FCC) grade) (65%, volume/volume (v/v)) in an aqueous solution denatured according to Alcohol and Tobacco Tax and Trade Bureau regulations in 27 CFR part 20.
- Glycerol (4.2% v/v).
- Aloe Barbadensis Leaf Extract.
- Sterile distilled water or boiled cold water.
- Fragrance
The finished product formulation underwent third party efficacy testing (kill study) to confirm efficacy and product potency.
The hand sanitizer come in 6 different scents. Fragrance free (submitted separately NDC 76751-211), Lavender (NDC 76751-611), Orange Blossom (NDC 76751-511), Mint Citrus (NDC 76751-111), Charcoal (NDC 76751-311) and Cucumber Basil (NDC 76751-411).
Active Ingredient(s)
Alcohol 65% v/v. Purpose: Antiseptic
Purpose
Antiseptic, Hand Sanitizer
Warnings
For external use only. Flammable. Keep away from heat or flame
Do not use
- in children less than 2 months of age
- on open skin wounds
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Other information
- Store between 15-30C (59-86F)
- Avoid freezing and excessive heat above 40C (104F)
Use
Helps reduce bacteria on skin.
Directions
- Remove nozzel bead.
- Spray liquid in hands and rub until dry.
- Use as often as needed.
Inactive Ingredients
Inactive Ingredients for NDCs 76751-111-01; 76751-311-01; 76751-411-01; 76751-511-01; 76751-611-01
- Aloe Barbadensis Leaf Extract
- Fragrance (Essential Oil Blend)
- Glycerin
- Water
Primary container spray bottle bottom label
Bottom label for primary container spray bottle for all scents:

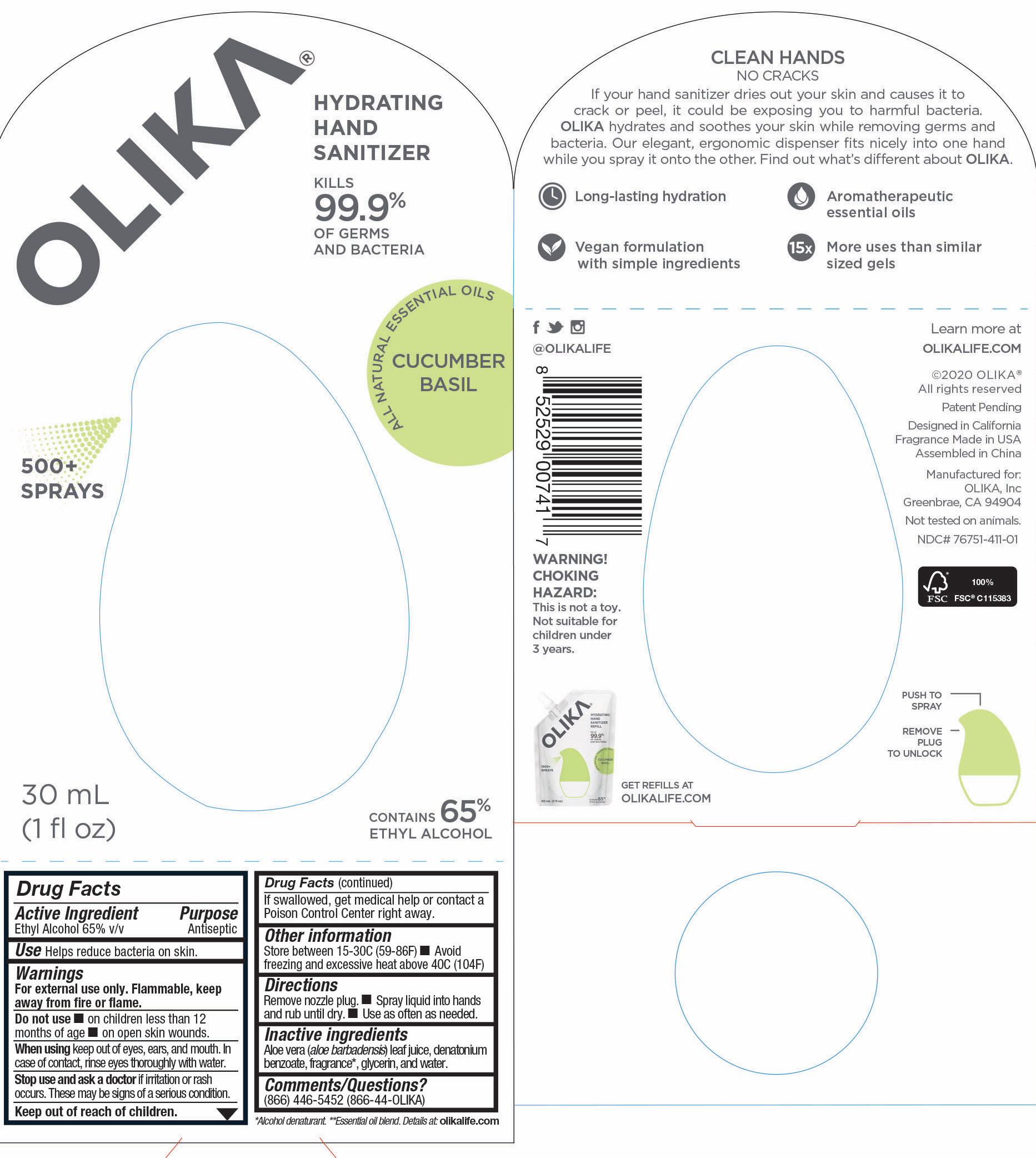
76751-411-01 Prinicipal Display Panel and information panel image
76751-411-01 30 mL
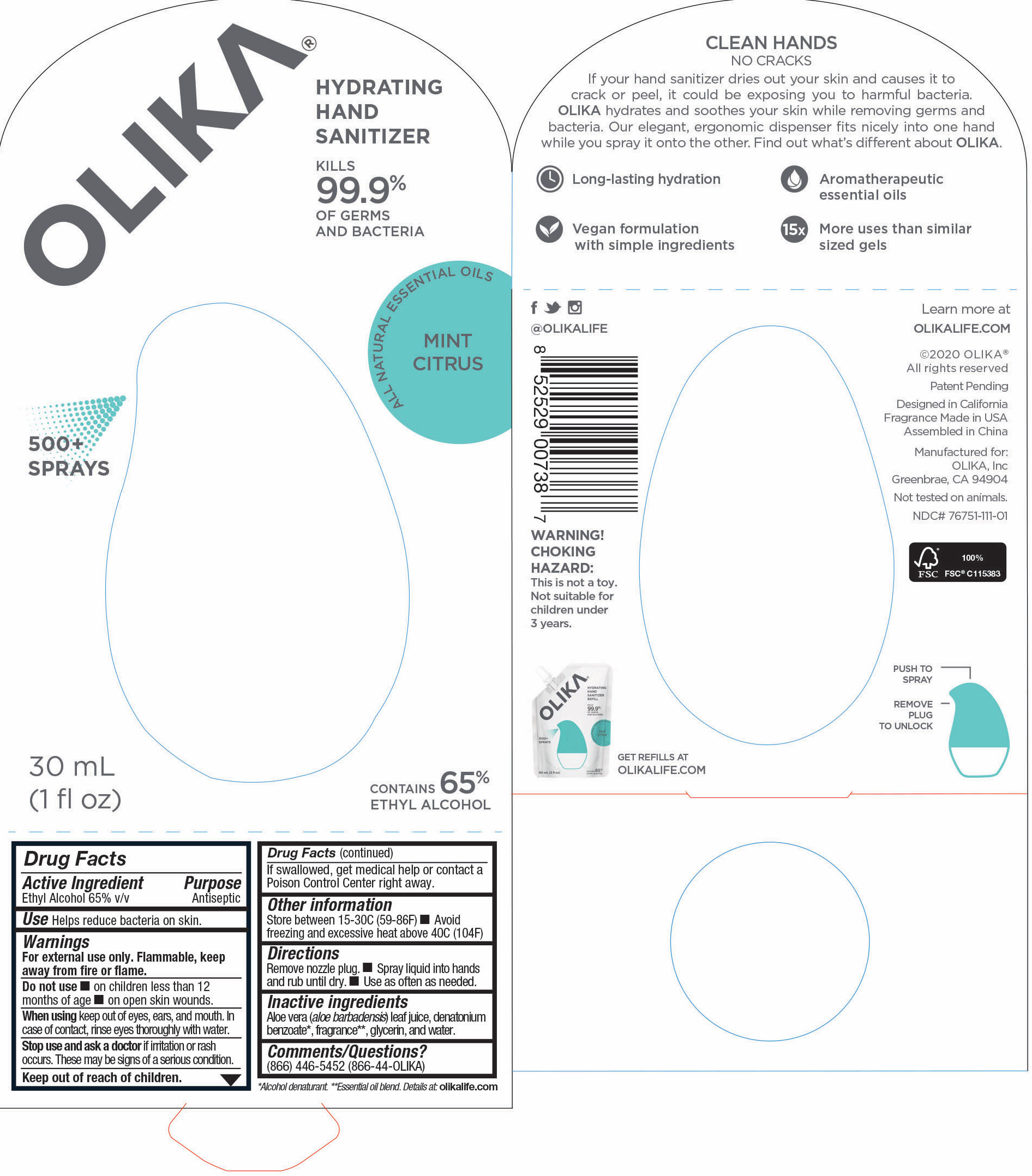
76751-111-01 Prinicipal Display Panel and information Panel
30 mL NDC 76751-111-01
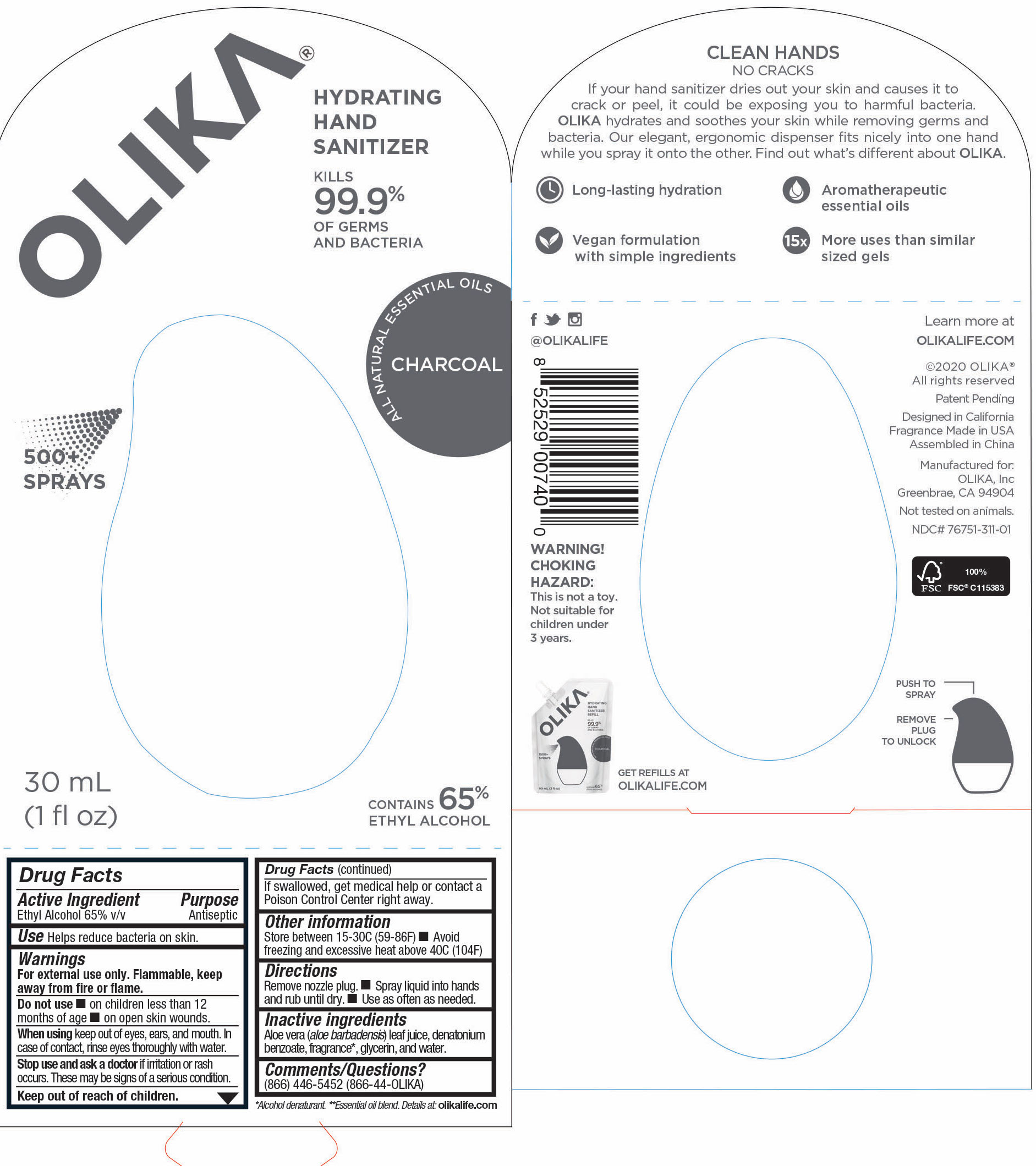
76751-311-01 Image Principal Display Panel & Information Panel
30 mL NDC 76751-311-01
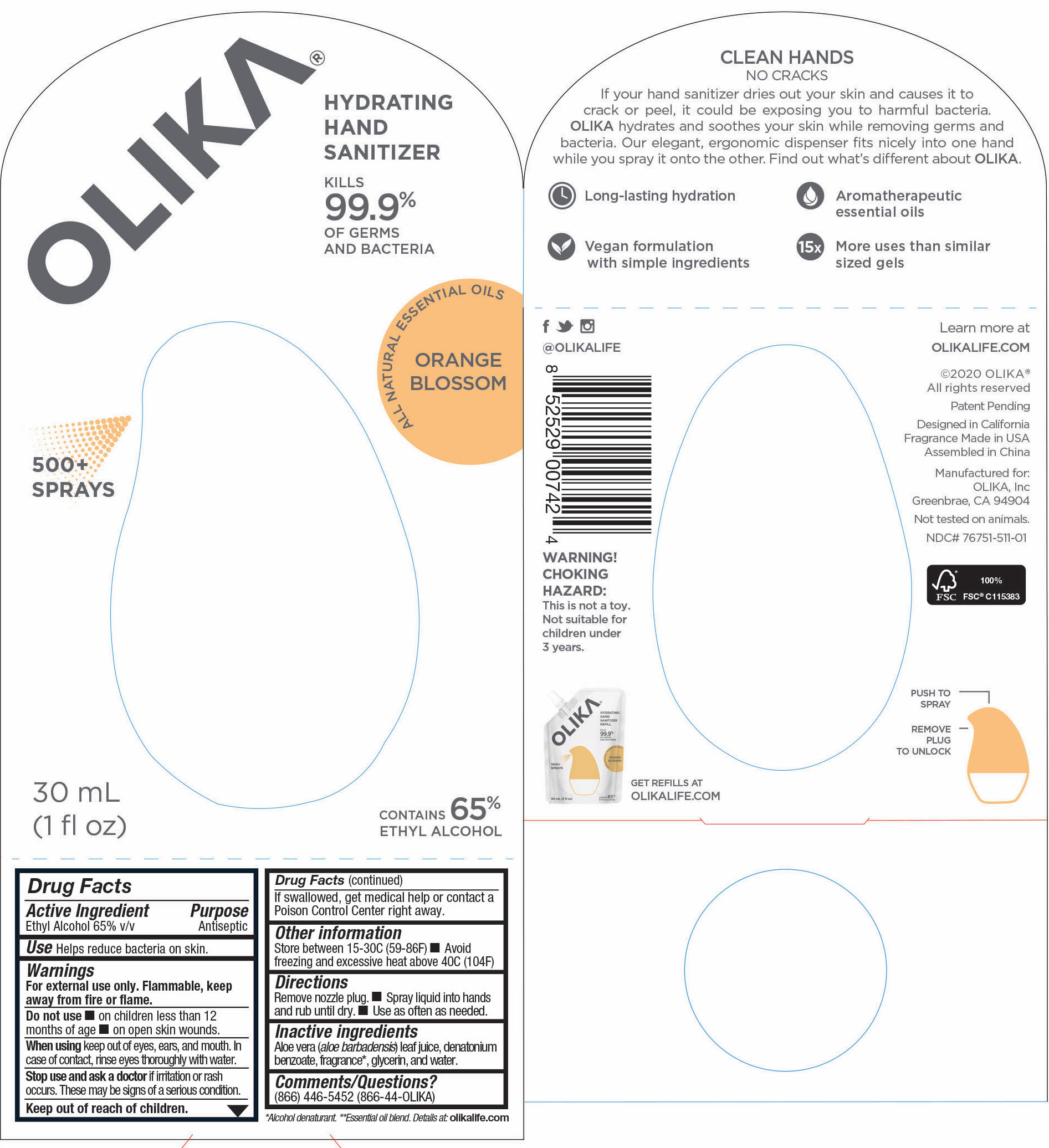
76751-511-01 Principal Display panel and information panel
30mL NDC 76751-511-01
76751-111-02 Principal Display and Information Panel
20 mL NDC 76751-111-02
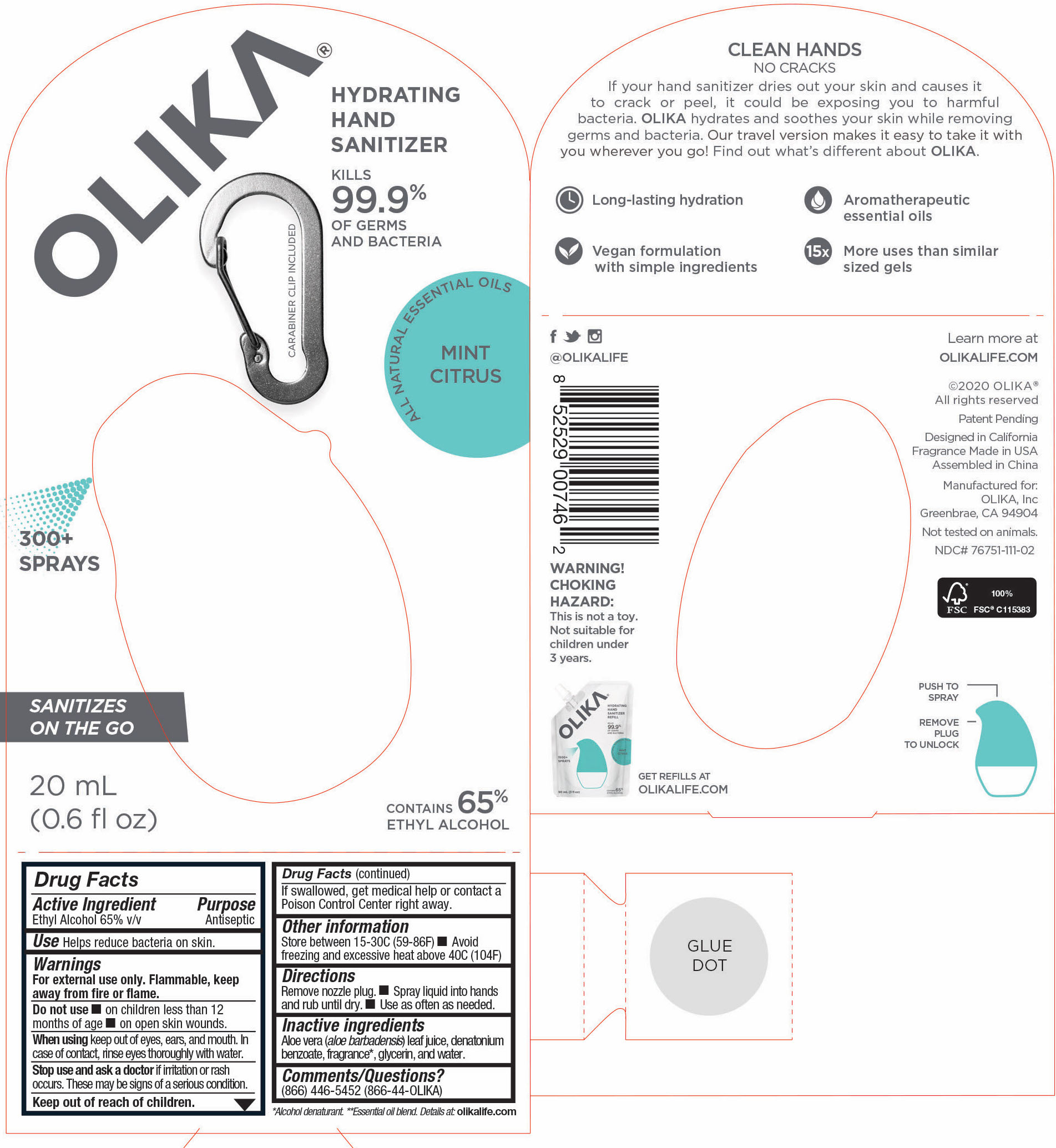
76751-311-02 Principal Display and Information Panel
20mL NDC 76751-311-02
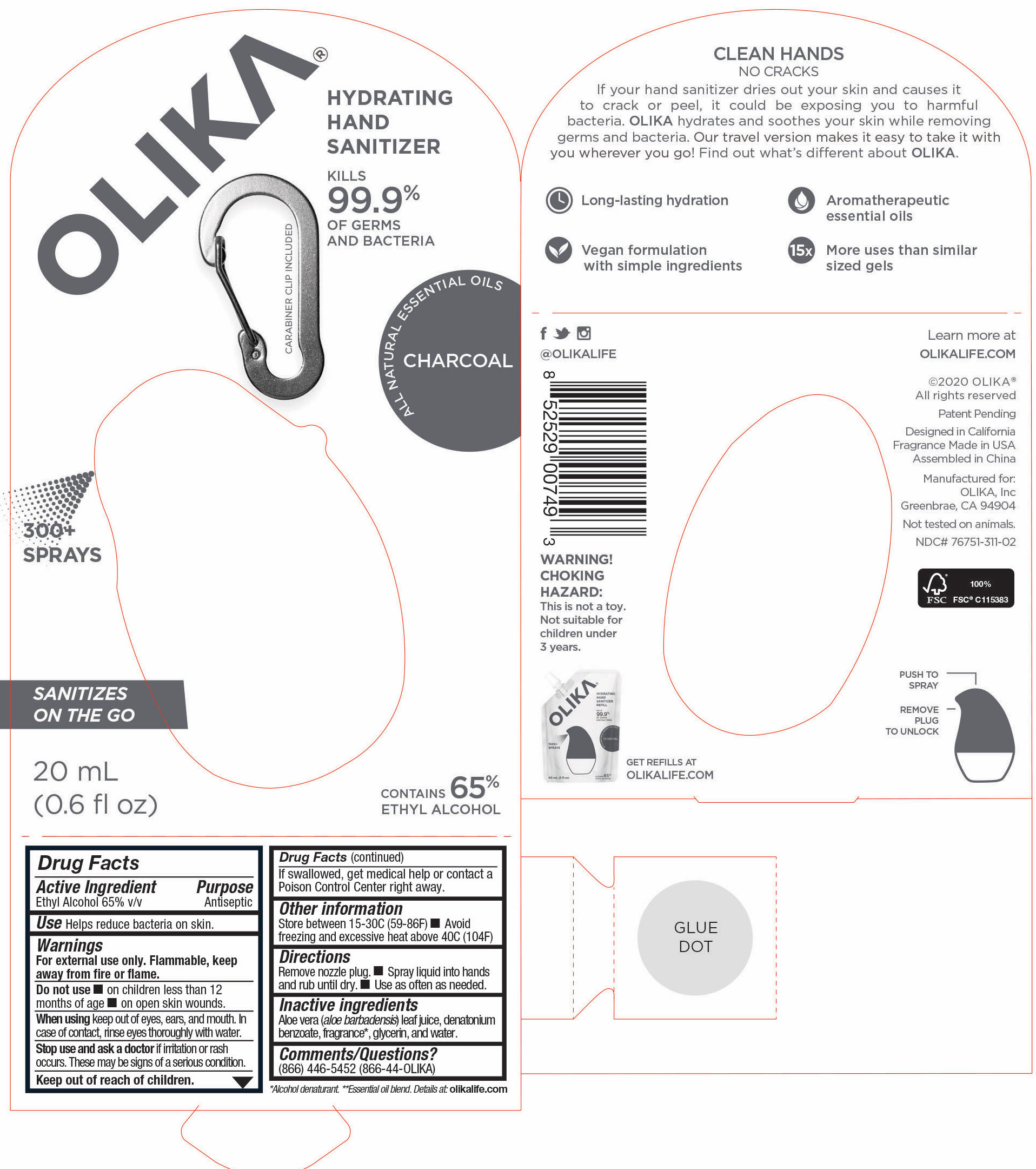
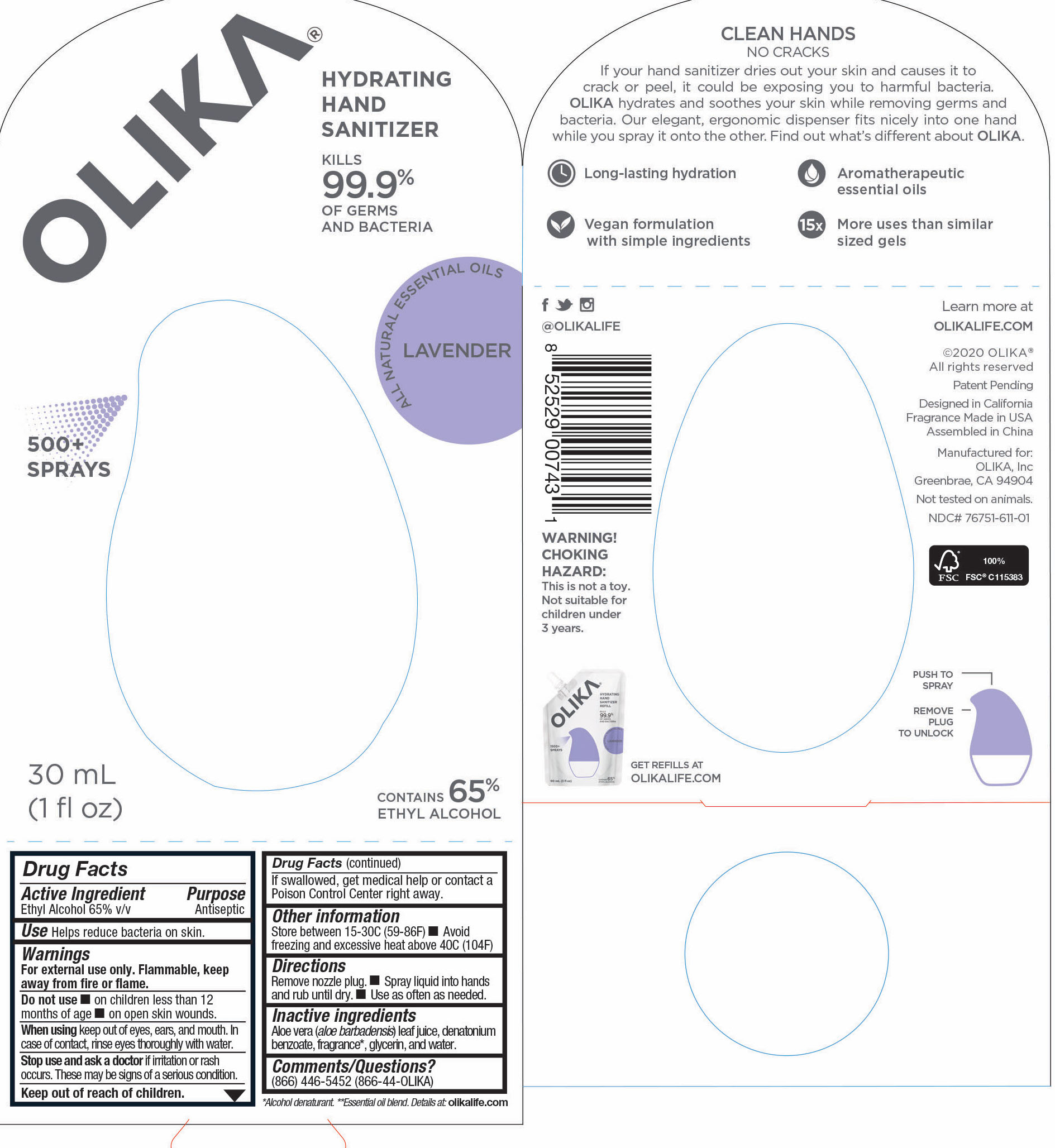
76751-611-02 PRINCIPAL DISPLAY PANEL AND INFORMATION LABEL
20 mL NDC 76751-611-02
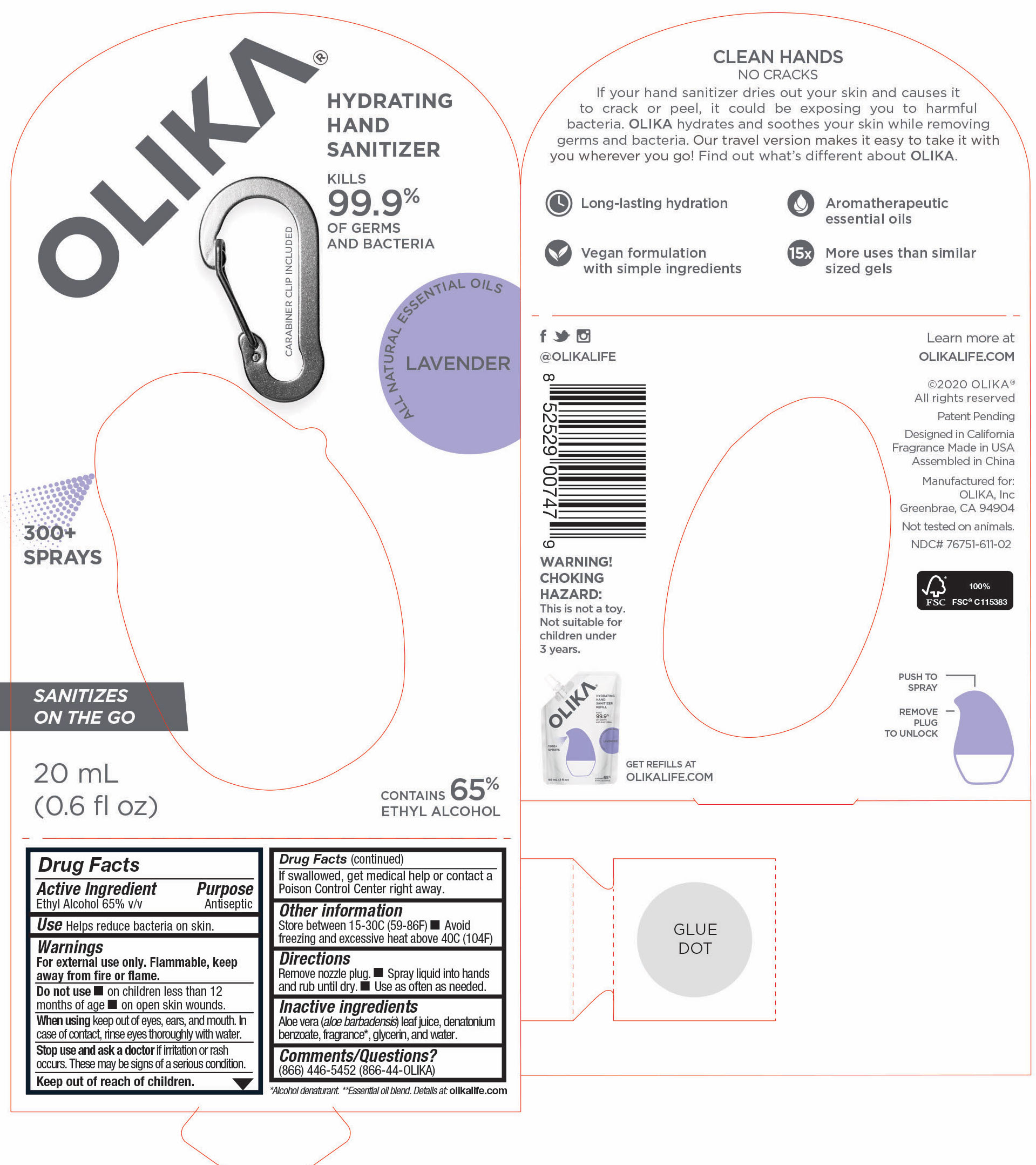
76751-411-02 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
20 mL NDC 76751-411-02
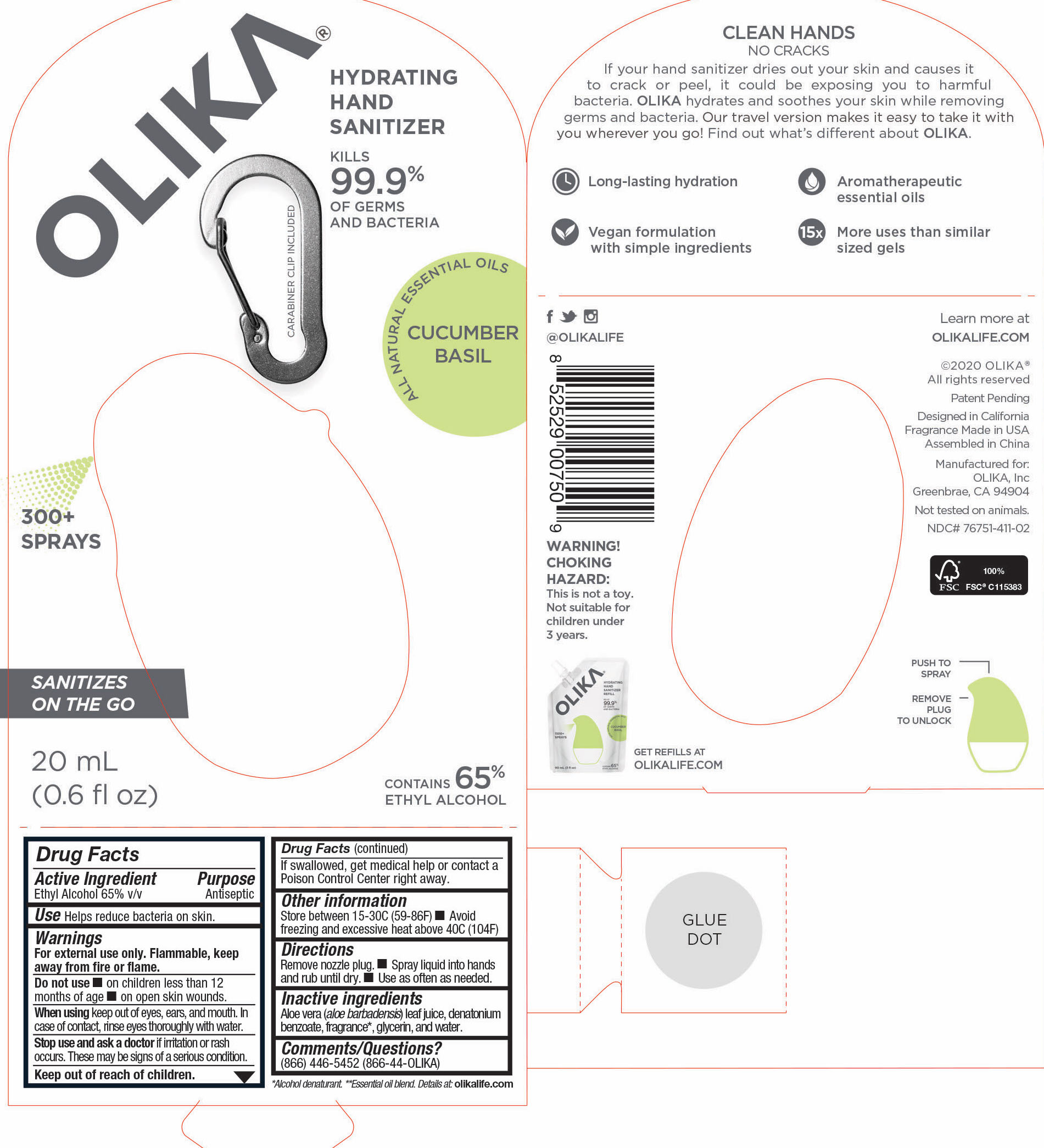
76751-111-03 PRINCIPAL DISCPLAY PANEL AND INFORMATION PANEL
90 mL NDC 76751-111-03

76751-611-03 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
90 mL NDC 76751-611-03

76751-311-03 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
90 mL NDC 76751-311-03

76751-411-03 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
90 mL NDC 76751-411-03

76751-511-03 PRINCIPAL DISPLAY PANEL AND INFORMATON PANEL
90 mL NDC 76751-511-03

76751-611-01 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
30mL NDC 76751-611-01
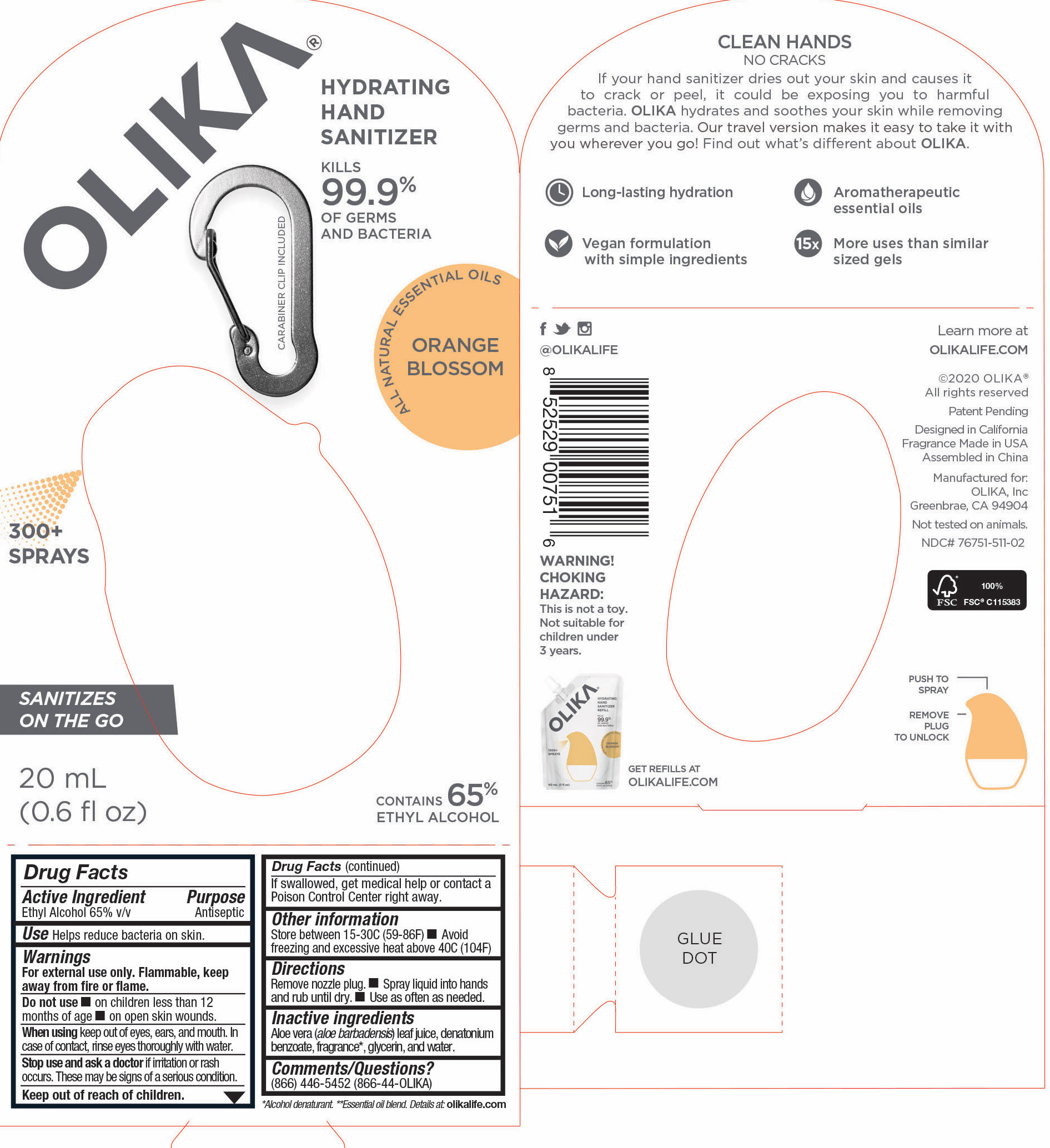
76751-511-02 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
20 mL NDC 76751-511-02
Olika Inc.















