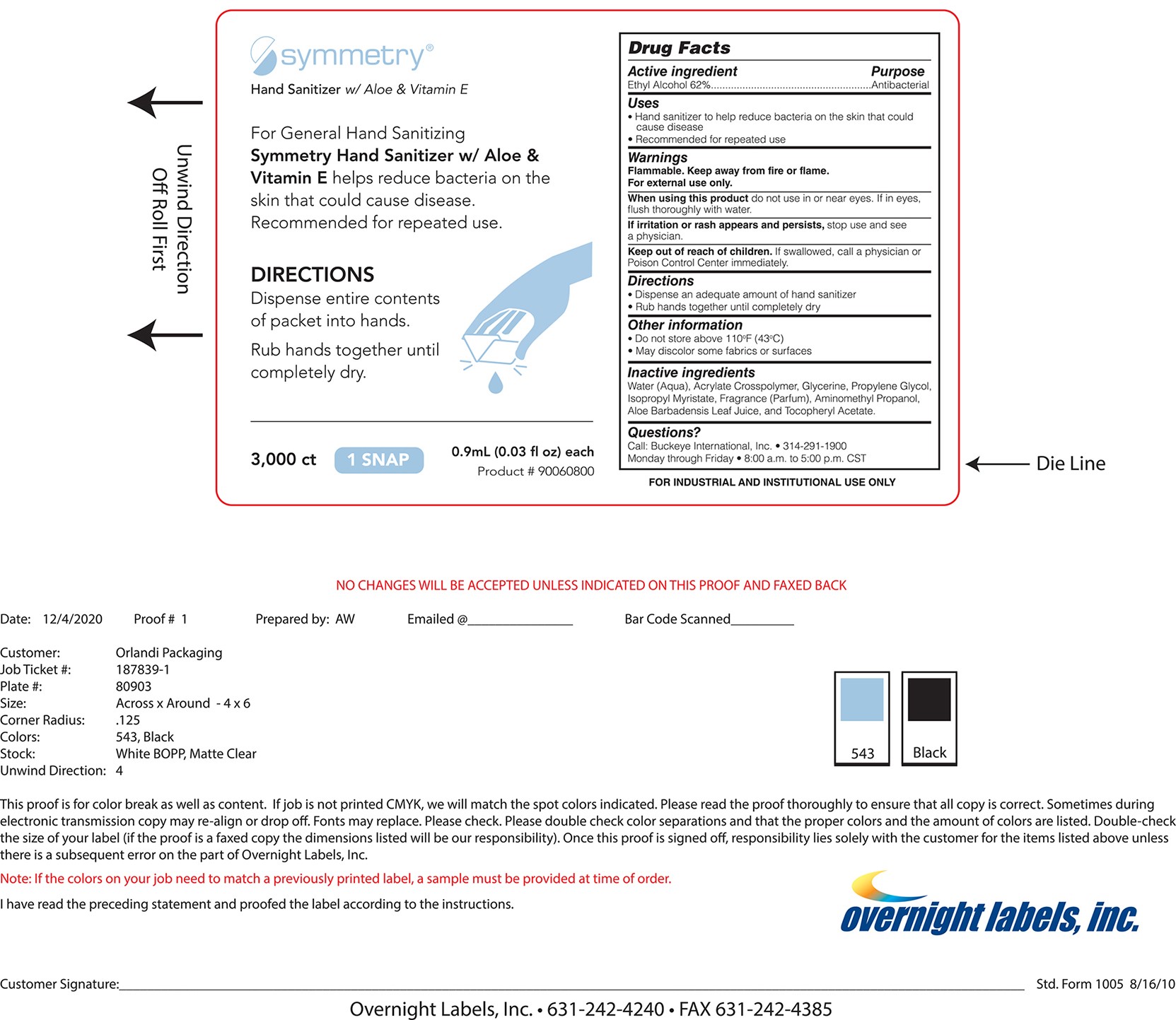
SYMMETRY HAND SANITIZER- hand sanitizer gel
Orlandi Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient(s)
Ethyl alcohol 62% Purpose - Antiseptic
Purpose
Antiseptic, Hand Sanitizer
Use
For hand washing to decrease bacteria on the skin that could casue disease
Warnings
For external use only
Flammable, keep away from fire or flame.
Do not use
in or near the eyes
Stop use and ask a doctor if
irritation and redness develop
condition persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Do not use
Keep out of reach of children. If swallowed, get medical help or contact a poison control center immediately.
For external use only. Do not use in the eyes.
Package Label - Principal Display Panel

Directions
- dispense entire contents of packet into hands. Rub hands together until dry.
Other information
Flammable, keep away from fire or flame.
Inactive ingredients
water (aqua), acrylate crosspolymer, glycerine, porpylene glycol, isopropyl myristate, fragrance (parfum), aminomethyl propanol, alor barnadensis juice, and tocopheryl acetate
Keep out of reach of children.
label
Orlandi Inc.

