CVSLIDOCAINEANTISEPTICSPRAY- lidocaine and benzalkonium chloride spray
CVS Health
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Temporarily relieves pain and itching due to: sunburn,minor burns,minor cuts,scrapes, insect bites,minor skin irritations,First aid to help kill germs.
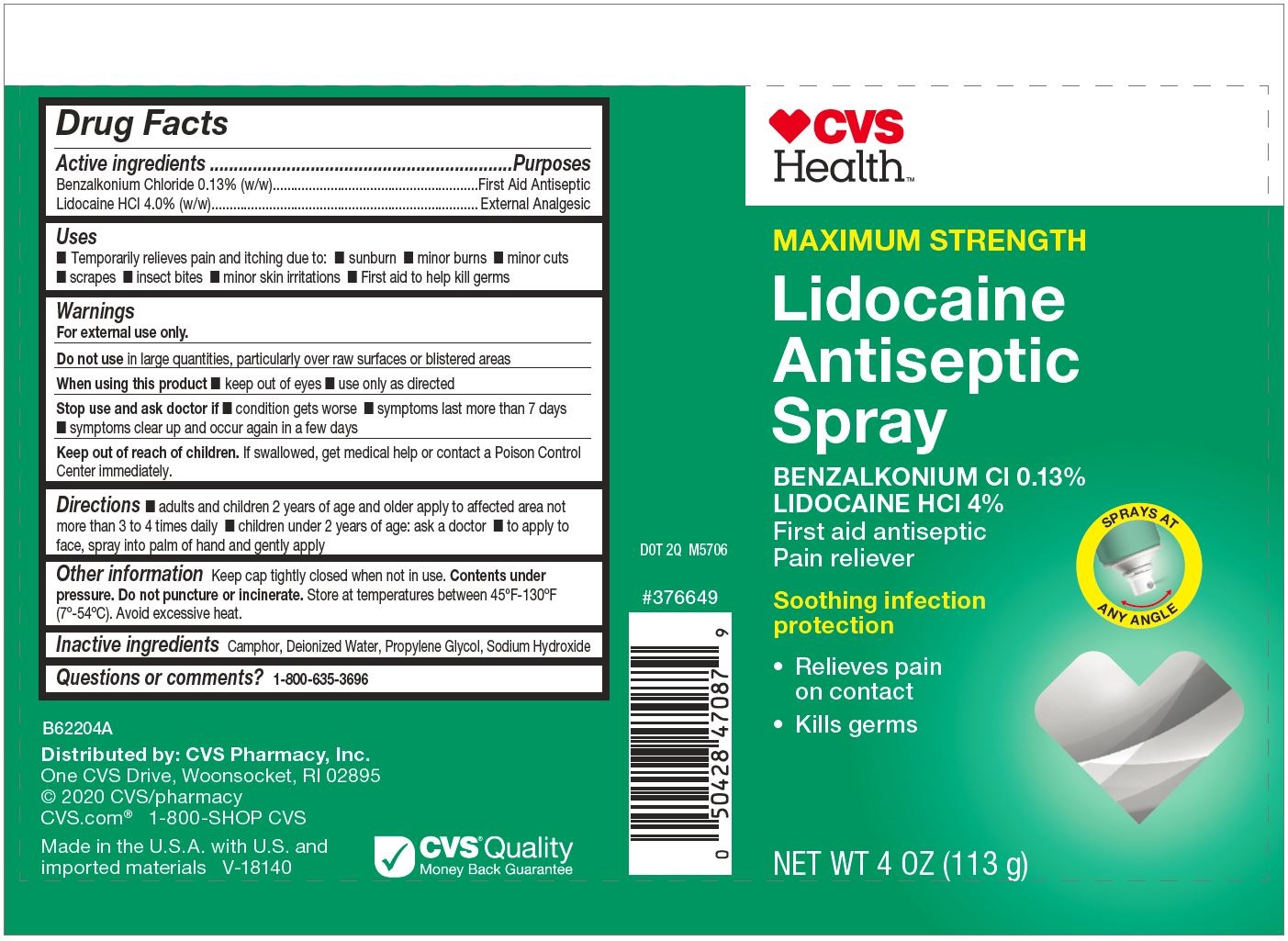
Drug Facts
Active ingredients
| Active ingredients | Purposes |
| Benzalkonium Chloride 0.13% (w/w) | First Aid Antiseptic |
| Lidocaine HCl 4.0% (w/w) | External Analgesic |
Uses
■ Temporarily relieves pain and itching due to: ■ sunburn ■ minor burns ■ minor cuts
■ scrapes ■ insect bites ■ minor skin irritations ■ First aid to help kill germs
Warnings
For external use only.
Do not use in large quantities, particularly over raw surfaces or blistered areas
When using this product ■ keep out of eyes ■ use only as directed
Stop use and ask doctor if ■ condition gets worse ■ symptoms last more than 7 days
■ symptoms clear up and occur again in a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control
Center immediately.
Directions
■ adults and children 2 years of age and older apply to affected area not more than 3 to 4 times daily ■ children under 2 years of age: ask a doctor ■ to apply to face, spray into palm of hand and gently apply
Other information
Keep cap tightly closed when not in use.
Contents under pressure. Do not puncture or incinerate. Store at temperatures between 45ºF-130ºF (7º-54ºC). Avoid excessive heat.
Inactive ingredients
Camphor, Deionized Water, Propylene Glycol, Sodium Hydroxide
Questions or comments?
1-800-635-3696
Principal Display Panel
CVS
Health
™
MAXIMUM STRENGTH
Lidocaine
Antiseptic
Spray
BENZALKONIUM Cl 0.13%
LIDOCAINE HCl 4%
First aid antiseptic
Pain reliever
SPRAYS
ANY ANGLE
Soothing infection
protection
on contact
NET WT 4 OZ (113 g)