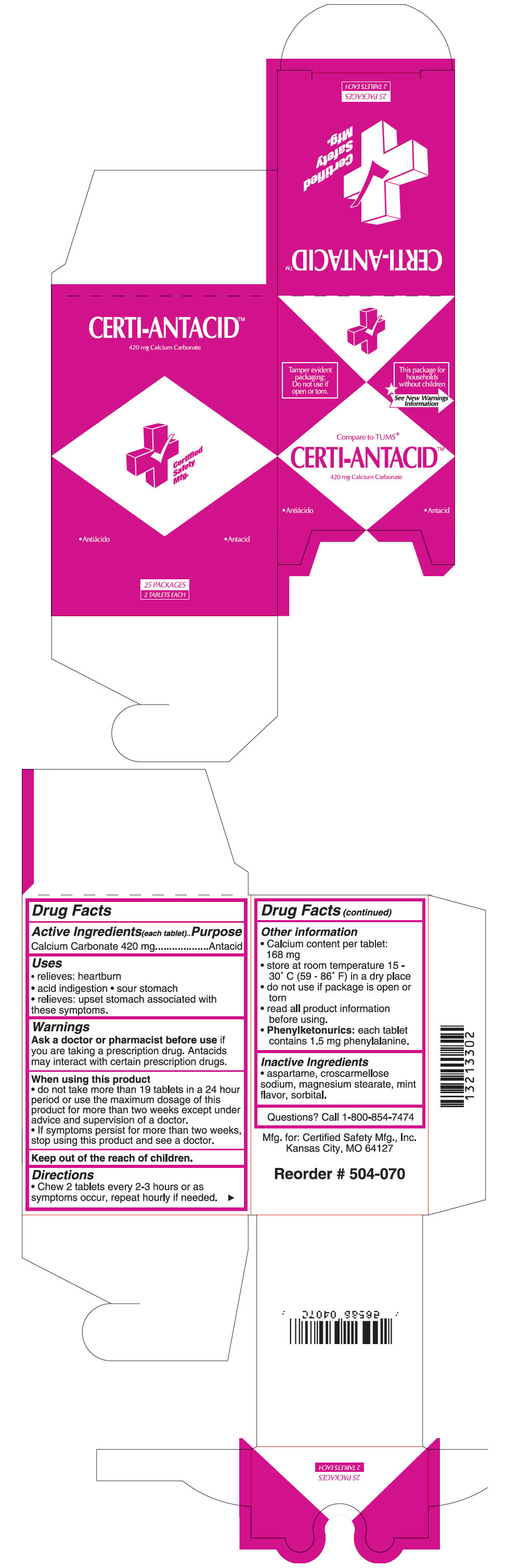
CERTI-ANTACID- calcium carbonate tablet
Certified Safety Manufacturing
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients(each tablet)
Calcium Carbonate 420 mg
Uses
- relieves: heartburn
- acid indigestion
- sour stomach
- relieves: upset stomach associated with these symptoms.
Warnings
Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
- do not take more than 19 tablets in a 24 hour period or use the maximum dosage of this product for more than two weeks except under advice and supervision of a doctor.
- If symptoms persist for more than two weeks, stop using this product and see a doctor.
Keep out of the reach of children.
Directions
- Chew 2 tablets every 2-3 hours or as symptoms occur, repeat hourly if needed.
Other information
- Calcium content per tablet: 168 mg
- store at room temperature 15 - 30° C (59 - 86° F) in a dry place
- do not use if package is open or torn
- read all product information before using.
-
Phenylketonurics: each tablet contains 1.5 mg phenylalanine.
Inactive Ingredients
- aspartame, croscarmellose sodium, magnesium stearate, mint flavor, sorbital.
Questions?
Call 1-800-854-7474
PRINCIPAL DISPLAY PANEL - 420 mg Tablet Pouch Box
Tamper evident
packaging:
Do not use if
open or torn.
This package for
households
without children
See New Warnings
Information
Compare to TUMS®
CERTI-ANTACID™
420 mg Calcium Carbonate
•Antacid