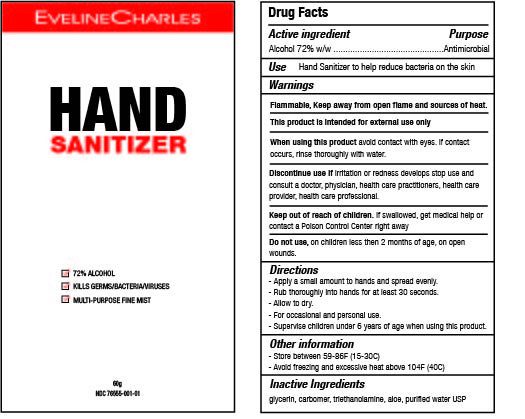
EVELINE CHARLES HAND SANITIZER- alcohol gel
Ec Labs Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Hand sanitize eliminates bacteria, germs and viruses. Skin softening formula features aloe vera and glycerin for hydration and protection.
Active Ingredient(s)
Alcohol 72% w/w. Purpose: Antimicrobial
Use
Hand Sanitizer to kill harmful germs/bacteria/viruses. For use when soap and water are not available.
Warnings
For external use only
Flammability warning. Keep away from open flame and sources of heat
When using this product avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Stop use and ask/ consult a doctor/ physician/ health care practitioner/ health care provider/ health care professional if irritation develops.
Keep out of reach of children. If swallowed, call a poison control centre or get medical help right away.
Do not use
- in children less than 2 months of age
- on open skin wounds
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply a small amount to the hands and spread evenly. Rub thoroughly into hands for at least 30 seconds. Allow to dry. For occasional and personal domestic use.
- Supervise children under 6 years of age when using this product to avoid swallowing.
Other information
- Store between 15-30C (59-86F)
- Avoid freezing and excessive heat above 40C (104F)
Inactive ingredients
glycerin, carbomer, triethanolamine, aloe, purified water USP
 60g NDC: 76555-001-01
60g NDC: 76555-001-01
240g NDC: 76555-001-02

296g NDC: 76555-001-03

500g NDC: 76555-001-04

750g NDC: 76555-001-05
1kg NDC:76555-001-06
4kg NDC:76555-001-07



 60g NDC: 76555-001-01
60g NDC: 76555-001-01





