DESCRIPTION
Lidocaine 4% cream is a non-greasy cream specially formulated with soothing agents, indicated as a topical anesthetic for use on normal intact skin for local analgesia and itching due to minor cuts, minor scrapes, sunburn, minor skin irritations, minor burns and insect bites.
INACTIVE:
Butylated Hydroxytoluene, Cetostearyl Alcohol, Citric Acid, Edetate Disodium, Light Mineral Oil, Methylparaben, Polyoxyl 20 Cetostearyl Ether, Propylene Glycol, Propylparaben, Purified Water, Sodium Citrate and White Petrolatum.
The chemical designation of Lidocaine is 2-(diethylamino)-N(2,6-dimethylphenyl), acetamide. The structure of lidocaine is:
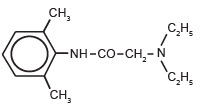
MECHANISM OF ACTION
Lidocaine 4% cream applied to intact skin provides dermal analgesia by the release of lidocaine from the cream into the epidermis and dermis. Lidocaine is a local anesthetic agent of the amide type. Local anesthetics reversibly block the initiation and conduction of nerve impulses by interfering with the flux of sodium ions through the neuronal membrane. The onset, depth and duration of dermal analgesia provided depend upon the site and duration of application.
INDICATIONS AND USAGE
Lidocaine 4% cream is indicated for use on normal intact skin for temporary relief of pain and itching due to minor cuts, minor scrapes, minor skin irritations, minor burns and insect bites.
Lidocaine 4% cream is not recommended for internal use, in the or near the eyes and in large quantities, particularly over raw surfaces or blistered areas.
CONTRAINDICATIONS
Lidocaine 4% cream is contraindicated in patients with sensitivity to amide type local anesthetics or to any component of the product.
WARNINGS
For external use only. Avoid contact with the eyes. Do not use over large areas of the body. Do not use for more than seven days unless directed by a doctor. Keep this and all drugs out of the reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Inappropriate use of this product, such as on large areas of the body, application on mucous membranes, or on individuals that are allergic to the amide type anesthetics, may result in serious side effects. Consultation with a doctor before using this product is strongly recommended.
PRECAUTIONS
Repeated doses of lidocaine 4% cream may increase blood levels of lidocaine. Avoid contact with the eyes. If eye contact occurs, immediately wash out the eye with water or saline.
The patient should be aware that dermal analgesia may be accompanied by the block of all sensations in the treated skin. For this reason, the patient should avoid inadvertent trauma to the treated area by scratching, rubbing or exposure to extreme hot or cold temperatures until complete sensation has returned.
DRUG INTERACTIONS
Lidocaine 4% cream should be used with caution in patients receiving Class I antiarrhythmic agents (e.g., tocainide, mexiletine) since the toxic effects are potentially additive and synergistic.
- Adults and children 2 years and older. Apply externally to the affected area up to 3 to 4 times a day.
- Children under 2 years of age. Consult a doctor.
