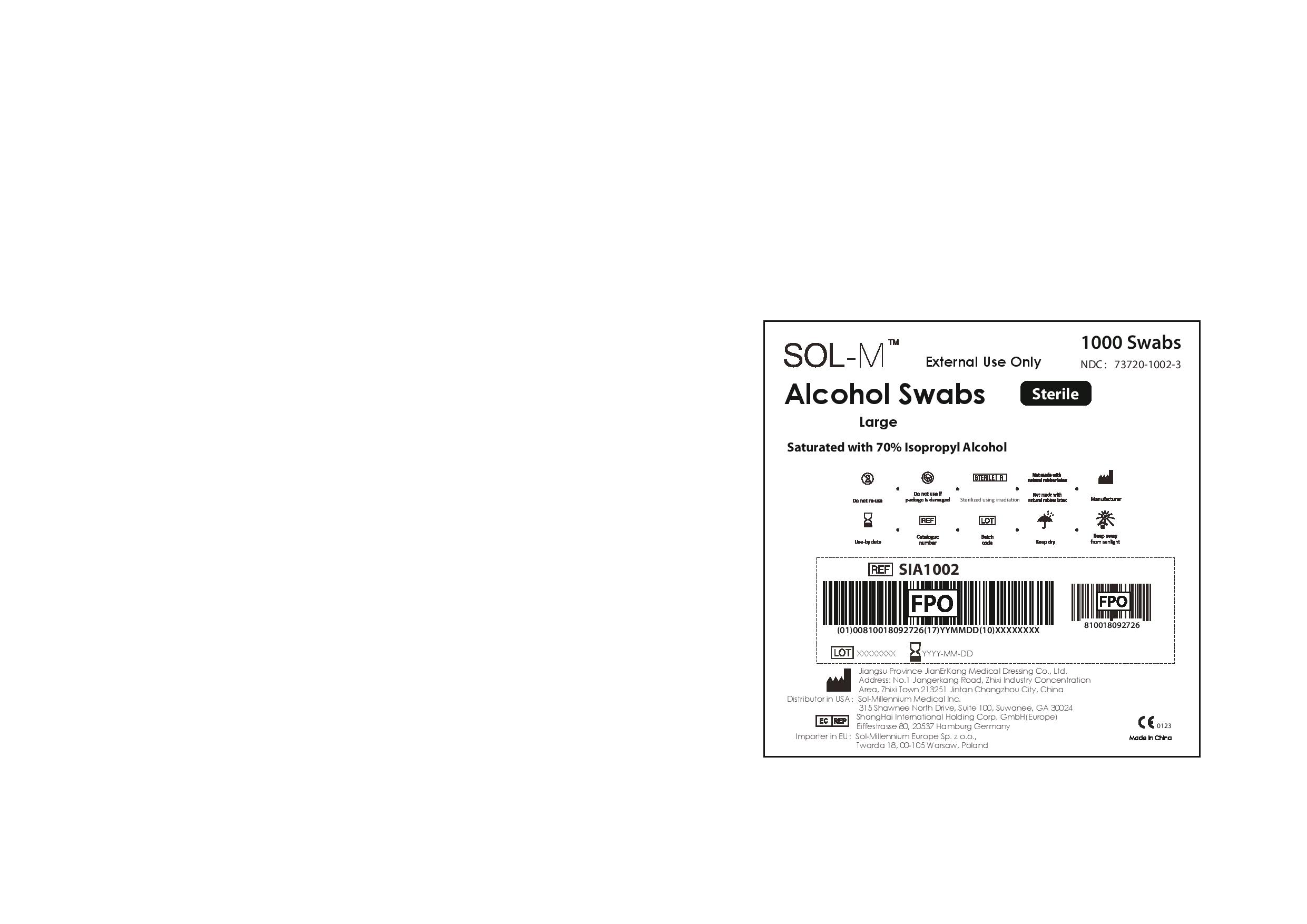
1000 Swabs
External Use Only
NDC: 73720-1002-3
Sol-M Alcohol Swabs Sterile
Large
Saturated with 70% Isopropyl Alcohol
Do not re-use
Do not use if package is damaged
Sterilized using irradiation
Not made with natural rubber latex
Manufacturer
Use-by date
Catalogue number
Batch code
Keep dry
Keep away from sunlight
Jiangsu Province JianErKang Medical Dressing Co., Ltd.
Address: No.1 Jangerkang Road, Zhixi Industry Concentration
Area, Zhixi Town 213251 Jintan Changzhou City, China
Distributor in USA: Sol-Millennium Medical Inc.
315 Shawnee North Drive, Suite 100, Suwanee, GA 30024
ShangHai International Holding Corp. GmbH(Europe)
Eiffestrasse 80, 20537 Hamburg Germany
Importer in EU: Sol-Millennium Europe Sp. zo.o.,
Twarda 18, 00-105 Warsaw, Poland