Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian. Contains a new animal drug for use only in investigational animals in clinical trials. Not for use in humans. Edible products of investigational animals are not to be used for food unless authorization has been granted by the U.S. Food and Drug Administration or by the U.S. Department of Agriculture.
Rev. 10-2015
Product Information
See WARNINGS concerning use in animals with certain conditions.
DESCRIPTION
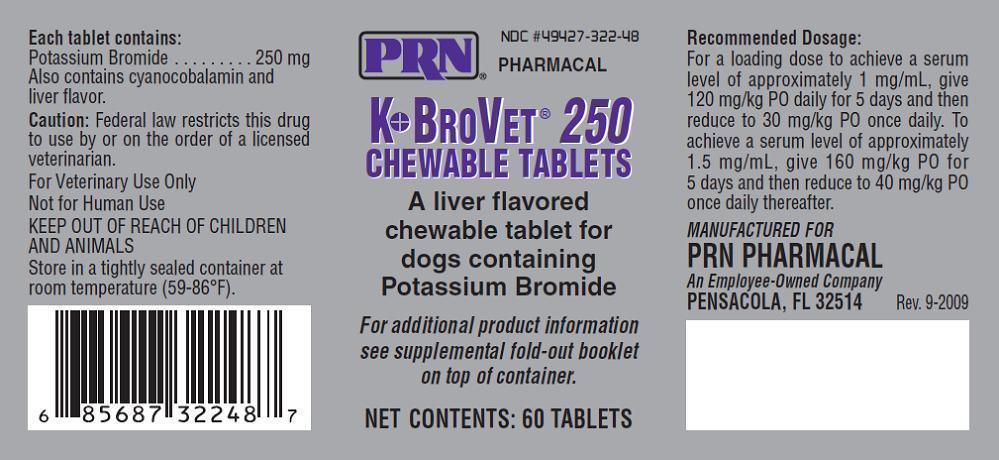
K●BroVET® Chewable Tablets are liver flavored tablets containing 250 mg or 500 mg of potassium bromide and cyanocobalamin.
Storage Conditions:
Store in a tightly sealed container at room temperature (59-86F).
Keep out of reach of children and animals.
INDICATION
K●BroVET® potassium bromide is used to treat seizure disorders in dogs. It can be used in addition to therapy with other agents or as the sole anticonvulsant.
CONTRAINDICATIONS
K●BroVET® should not be used in animals with a history of hypersensitivity to Bromide or any of the com-ponents of the solution or tablets.
DOSAGE AND ADMINISTRATION
Recommended Dosage for K●BroVET® Oral Solution and Tablets:
To achieve a serum level of approximately 1 mg/mL, give 120 mg/kg PO daily for 5 days then reduce to 30mg/kg PO once daily. To achieve a serum level of approximately 1.5 mg/mL, give 160 mg/kg PO for 5 days and then reduce to 40 mg/kg PO once daily thereafter.{R-21}
Among dogs responses to bromide may vary, treatment should be tailored to the individual dog, based on serum bromide concentration, success of seizure control, and the observation of adverse effects. Steady state serum concentrations generally take 4 to 5 months in dogs; therapeutic concen-trations may be reached before steady state at about 4 weeks after the beginning of treatment, serum bromide concentration may be tested to assess the response to product and, if results are satisfactory, the next testing for steady state serum levels is typically about 4 months. If the dog’s serum concentration is within range and the dog is doing well in the therapeutic range, serum concentration may then be tested every 6 to 12 months. Serum bromide is typically evaluated at the end of the loading period, then as described above when an animal is started with a loading dose.
Dogs― An oral dose of 30 mg of Bromide per kg of body weight every twelve hours for 115 days produced a steady state serum concen- tration of 2.45 mg/mL (range, 1.78 to 2.69 mg/mL) in Beagles fed a diet contain-ing 0.55% chloride.{R-3}
WARNINGS
This medication should be used cautiously in older animals as they will be more susceptible to adverse effects. This medication is not indicated for use in cats.
Possible Side Effects:
Dog may experience drowsi-ness when taking , but this will generally go away after approximately 3 weeks. Increased hunger, thirst, urination, vomiting, constipation, anorexia, and uncoordinated movements may occur with . During the load in dose increased nausea may be experienced.
There is no information on the relative frequency of pan-creatitis in dogs associated with bromide therapy alone. However, pancreatitis has been reported to be more frequent in dogs on concurrent phenobarbital and bro-mide therapy than dogs on phenobarbital alone.{R-12}
Personality changes have been occasionally reported in dogs on bromide, including attention seeking, irritability or aggression, and aimless pacing.
Reproduction/Pregnancy/Lactation
The effects of bromide on canine reproduction have not been studied.
Young Dogs
Safety of administering bro-mide to neonates and young animals has not been evaluated.{R-16-17}
Drug interactions and/ or related effects
Drug interactions and/or related effects have been selected on the basis of their clinical significance (possible mechanism in parentheses where appropriate) ― not necessar-ily inclusive (»=major clinical significance):
Note: Any of the following medications taken in combinations, depending on the amount taken, could also interact with K●BroVET®
Medications and foods containing chloride, bromide and chloride compete for reabsorption by the kidneys; increased amounts of chloride can promote loss of bromide in the urine, leading to a lowering of serum bromide concentrations: Decreased chloride consumption will promote increased renal reabsorption of bromide.{R-1; 2; 4; 5; 7; 15}
Halothane anesthesia (when inhaled a percentage of halothane is metabolized by dogs to produce bromide, along with other compounds; peak serum concentration occurs within about a day and, in one group of dogs, ranged from 0.04 to 0.088 mg/mL and persisted, with some diminishment, for at least ten days.{R-10; 11} Consideration should be given to animals that must have repeated anesthesia or that already require high serum bromide concentration for seizure control. Increased bromide levels due to metabolism of halothane are unlikely to be a significant risk for bromide toxicity in most dogs.
Mechanism of action/Effect:
Bromide's mechanism of action has not been clearly defined. By preferential movement across neuronal membranes via gamma-aminio butyric (GABA) - activated chloride channels, bromide may aid in controlling seizures through hyperpolarization of neuronal cell membranes, leading to stabilization and decreased sensitivity to epileptic foci. {R-2; 15}
Volume of distribution
Dogs: 0.45 ± 0.07 L/kg{R-4}
Protein binding: Bromide is minimally protein bound.{R-20}
Biotransformation: Bromide is not biotransformed by the liver and is eliminated unchanged, primarily by renal clearance.{R-2}
Half-life: Elimination ―
Bromide is freely filtered by the glomerulus, but is reabsorbed by the kidneys, in competition with chloride.{R-7}
Bromide reabsorption will predominate in the absence of a large chloride load, causing a significantly extended elimination half-life in dogs.{R-4; 5; 7} Increasing chloride intake by an animal on a low chloride diet will decrease the half-life of elimination.{R-14; 15}
Elimination: Renal. Because bromide is widely distrib-uted, minute amounts will also be excreted in saliva, sweat and feces.{R-5; 6} Rate of elimination of bromide will increase in dogs that receive a high level of chlo-ride supplementation.{R-4}
OVERDOSE
For more information in cases of overdose or unintentional ingestion, contact the American Society for the Prevention of Cruelty to Animals (ASPCA) National Animal Poison Control Center (888-426-4435 or 900-443-0000; a fee may be required for consultation) and/or the drug manufacturer.
Note: Signs of bromide toxicity are dose-related but dogs differ in their sensitivity. Bromide toxicity has rarely been reported in dogs with serum bromide concentrations of less than 1.5 mg/mL. Signs have been reported in some dogs with relatively low serum concen-tration (2.75 mg/mL) while not appearing in other dogs with significantly higher concen-tration (4 mg/mL).{R-3; 18} Dogs on concurrent bromide and phenobarbital therapy may be prone to bromide toxicity at lower serum concentrations than when bromide is administered alone. Other physical factors may also play a role in sensitivity.{R-3}
Short-term bromide toxicity is considered completely reversible.{R-18} However, mild bromide toxicity can progress to more severe nervous sys-tem dysfunction with ongoing high serum concentration of bromide.{R-8}
Other species ― Bromide toxicity has also been reported in cattle, goats and horses fed hay that contained bromide ion residue from accidental treatment with methyl bromide. Signs included ataxia, hind limb weakness, joint swelling, and sedation or recumbency.{R-9}
Clinical effects of overdose
Clinical signs may appear with serum bromide concen-tration >1.5 mg/mL in dogs on bromide therapy alone, but become more common as serum concentration increases, and most com-mon when serum concen-tration exceeds 4 mg/mL in dogs receiving bromide as the only anticonvulsant (2 to 3 mg/mL in dogs receiving concurrent phenobarbital and bromide).{R-3; 18}
Ataxia; diarrhea; hematochezia; salivation, excessive; shivering; skin lesions; stupor, progressing to coma and death – Reported with a dose of 200 to 500 mg/kg a day for 4 to 26 weeks.{R-13}
Treatment of bromide toxicity
Recommended treatment consists of the following:
For dogs on concurrent phenobarbital and bromide therapy with mild sedation, ataxia or hind limb weak-ness, a 10 to 25% reduction in phenobarbital may resolve the signs within five to seven days.{R-19}
For dogs receiving bromide as the only anticonvulsant, K●BroVET® may be discontinued for a few days while monitoring serum concentration, to see if mild signs of toxicity will resolve.
If necessary, renal excretion of bromide may be accelerated by increasing sodium chloride consumption or administering 0.9% sodium chloride intravenously over twelve hours, depending on the severity of the toxicity.R-7; 8; 13}
Supportive treatment
Continue to monitor
References
1. Podell M, Fenner WR. Bromide therapy in refractory canine idiopathic epilepsy. J Vet Intern Med (1993) Sep-Oct. 7(5):318-27.
2. Trepanier, L.A. Use of bromide as an anticonvulsant for dogs with epilepsy. J Am Vet Med Assoc. 1995 Jul 15, 207(2) 163-6.
3. March PA, Podell M, Sams RA.Pharmacokinetics and toxicity of bromide following high-dose oral potassium bromide administration in healthy beagles. J Vet Pharmacol Ther 2002, 25:425-32.
4. Trepanier LA, Babish JG. Pharmacokinetic properties of bromide in dogs after intravenous and oral administration of single doses. Res Vet Sci 1995, 58 248-51.
5. Wolf RL., Eadie GS. Reabsorption of bromide by the kidney. AM S Physiol 1950. 163(2) 436-41.
6. USP Committee comment. Rec 9/9/04.
7. Palmer JW, Clarke HT. The elimination of bromides from the blood stream. J Biol Chem 1933; 99:435.
8. Yohn SE, Morrison WB, Sharp PE. Bromide toxicosis (bromism) in a dog treated with potassium bromide for refractory seizures. J AM Vet Med Assoc 1992 Aug 1, 201(13) 468-70.
9. Knight HD, Costner GC. Bromide intoxication of horses, goats and cattle. J Am Vet Med Assoc 1977 Sept 1; 171(5):446-8.
10. Pedersoli WM. Serum bromide concentrations during and after halothane anesthesia in dogs. Am J Vet Res 1980 Jan; 41(1) 77-80.
11. De Moor A, Van Den Hende C, Moens Y et al. Increased plasma bromide concentrations in the horse after halothane anesthesia. Am J Vet Res 1978 Oct. 39(10): 1624-6.
12. Gaskill CL, Cribb AE. Pancreatitis associated with potassium bromide/phenobarbital combination therapy in epileptic dogs. Can Vet L 2000 Jul, 4: 555-8.
13. Nichols ES, Trepanier LA, Linn K. Bromide toxicosis secondary to renal insufficiency in an epileptic dog. J Am Vet Med Assoc 1996 Jan 15, 208(2) 231-3.
14. Shaw N, Trepanier LA, Garland S. High dietary chloride content associated with loss of therapeutic serum bromide concentrations in an epileptic dog. J Am Vet Med Assoc 1996 Jan15.208 (2) 234-6.
15. Trepanier LA, Babish JG, Effect of dietary chloride content on the elimination of bromide by dogs. res vet Sci 1995; 58: 252-5.
16. Okuda K, Yasuhhara A, Kamei A, et al. Successful control with bromide of two patients with malignant migrating partial seizures in infancy. Brain Dev 2000 Jan; 22(1): 56-9
17. Ernst JP, Doose H, Baier WK. Bromides were effective in intractable epilepsy with generalized tonic-clonic seizures and onset in early childhood. Brain Dev 1988;10(6) 385-8.
18. Sisson A. Current experiences with anticonvulsants in dogs and cats. In: Proceedings of the fifteenth annual veterinary medical forum. Lakewood, Colorado: American College of Veterinary Internal Medicine 1997. p. 596-8.
19. Trepanier LA. Optimal bromide therapy and monitoring. In:Proceedings of the fifteenth annual veterinary medical forum. Lakewood, Colorado: American College of Veterinary Internal Medicine 1997. p. 100-101.
20. USP Committee comment, Rec 11/01/04.
21 Plumbs veterinary Handbook 6th Edition.