Active ingredients** (in each tablet)
Arnica montana 4C HPUS (0.5mg)
Cimicifuga racemosa 4C HPUS (0.5mg)
Glonoinum 4C HPUS (0.5mg)
Lachesis mutus 5C HPUS (0.5mg)
Sanguinaria canadensis 4C HPUS (0.5mg) (contains less than 10 -9 mg chelidonine alkaloids)
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
Purpose*
Arnica montana 4C HPUS (0.5mg) ... Relieves red, blotchy face associated with hot flashes
Cimicifuga racemosa 4C HPUS (0.5mg) ... Relieves mood changes, irritability, and occasional sleeplessness associated with menopause
Glonoinum 4C HPUS (0.5mg) ... Relieves sudden hot flashes with profuse sweating and throbbing headache associated with menopause
Lachesis mutus 5C HPUS (0.5mg) ... Relieves hot flashes, night sweats, irritability, and mood changes associated with menopause
Sanguinaria canadensis 4C HPUS (0.5mg) ... Relieves flushing of the face associated with menopause
Uses*
temporarily relieves symptoms associated with perimenopause and menopause such as:
- hot flashes
- red, blotchy or flushed face
- night sweats
- mood changes
- irritability
- occasional sleeplessness
temporarily reduces the intensity and frequency of hot flashes associated with perimenopause and menopause
Keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults: At the onset of symptoms, day or night, allow 1 tablet to slowly dissolve in the mouth up to 4 times in 24 hours. Daily use for 3 months is recommended.
Children: Not recommended.
Do not use if glued carton end flaps are open or if the blister seal is broken.
Store below 86° F (30° C)
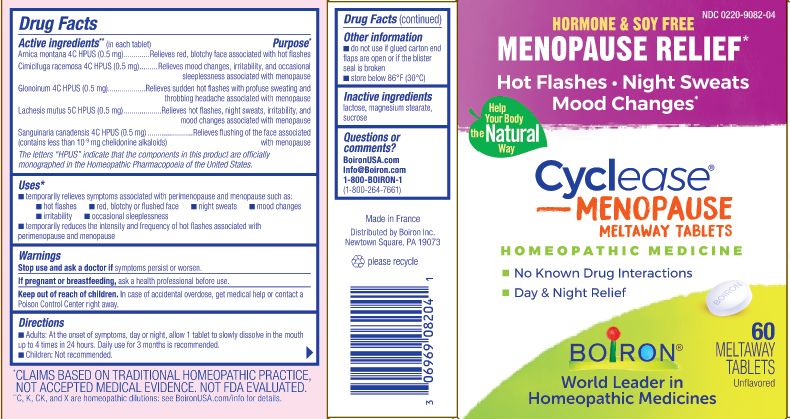
60 meltaway tablets unflavored
Hormone & Soy Relief
Menopause relief*
Hot Flashes Night Sweats Mood Changes*
No Known Drug Interactions
Day & Night Relief
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.