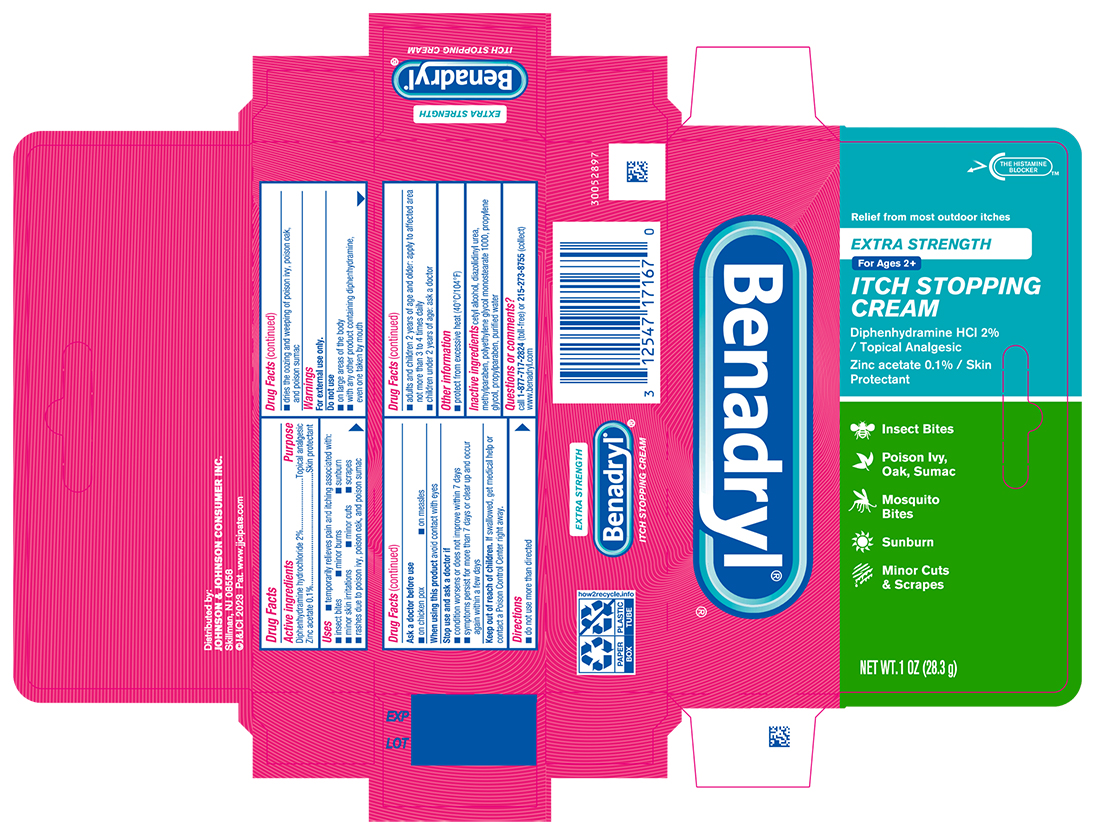
Uses
- temporarily relieves pain and itching associated with:
- insect bites
- minor burns
- sunburn
- minor skin irritations
- minor cuts
- scrapes
- rashes due to poison ivy, poison oak, and poison sumac
- dries the oozing and weeping of poison ivy, poison oak, and poison sumac
Warnings
For external use only.
Do not use
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
Directions
- do not use more than directed
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
Inactive ingredients
cetyl alcohol, diazolidinyl urea, methylparaben, polyethylene glycol monostearate 1000, propylene glycol, propylparaben, purified water
PRINCIPAL DISPLAY PANEL - 28.3 g Tube Carton
Benadryl® ®
THE HISTAMINE
BLOCKER TM
Relief from most outdoor itches
EXTRA STRENGTH
For Ages 2+
ITCH STOPPING
CREAM
Diphenhydramine HCl 2%
/ Topical Analgesic
Zinc acetate 0.1% / Skin
Protectant
Insect Bites
Poison Ivy,
Oak, Sumac
Mosquito
Bites
Sunburn
Minor Cuts
& Scrapes
NET WT.1 OZ (28.3g)