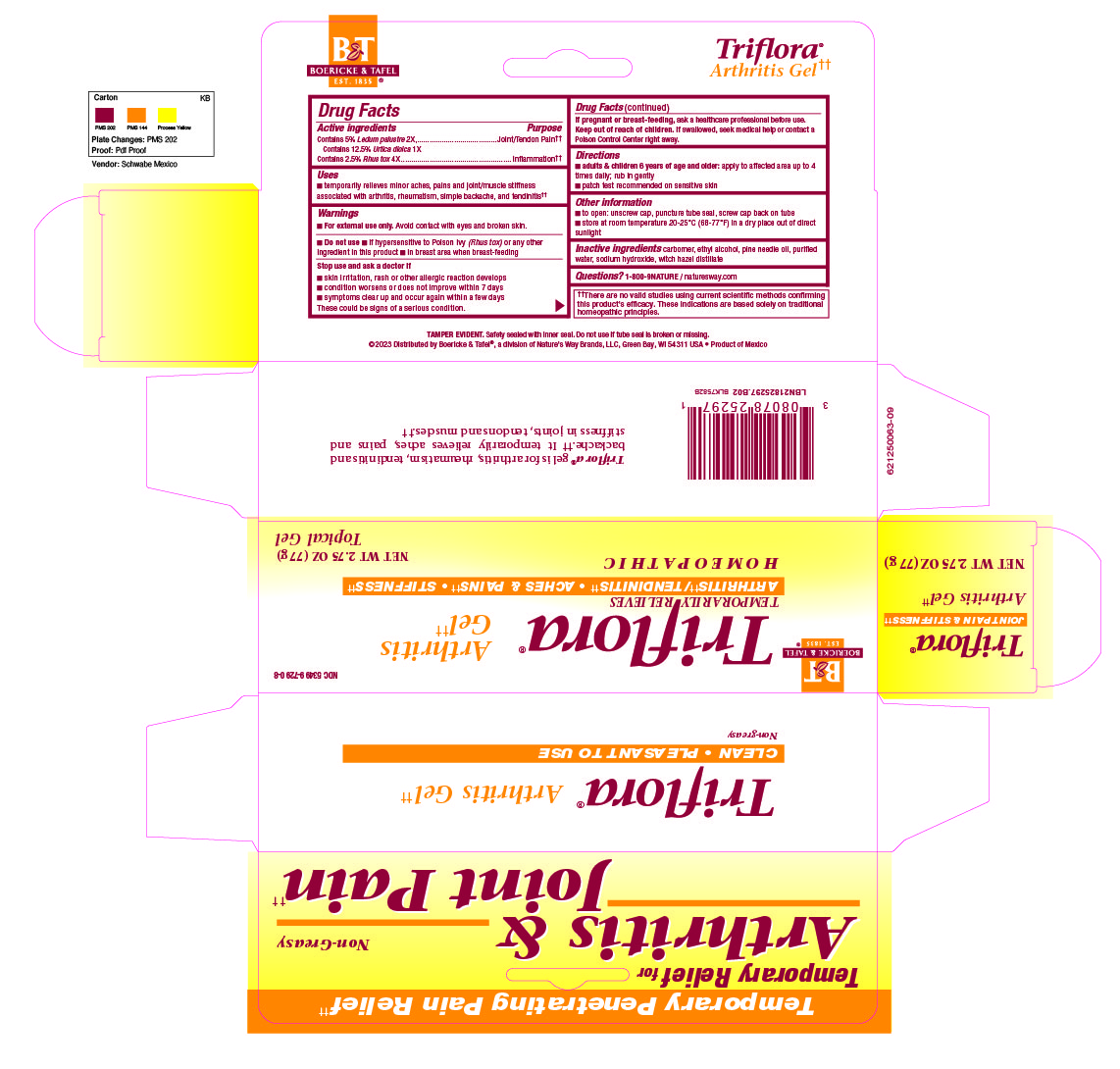
Active Ingredients
Contains 5% Ledium palustre 2X
Contains 12.5% Urtica doica 1X
Contains 2.5 Rhus tox 4X
Inactive Ingredients
carbomer
ethyl alcohol
pine needle oil
purified water
sodium hydroxide
witch hazel distillate
Dosage and Administration
Directions
Adult and children 6 years of age and older: apply to affected area up to 4 times daily. Rub in gently.
Patch test recommended on sensitive skin.
Indications and Usage
Temporarily relieves minor aches, pains, and joint/muscle stiffness associated with arthritis, rheumatism, simple backache and tendinitis.
Purpose
Temporarily relieves minor aches, pains, and joint/muscle stiffness associated with arthritis, rheumatism, simple backache and tendinitis.
Do Not Use
Do not use if hypersensitive to Posion Ivy (Rhus tox) or any other ingredients in this product, in breast area when breast-feeding.
Stop Use
Stop use and ask a doctor if skin irritation, rash or other allergic reaction develops, condition worsens or does not improve within 7 days, symptoms clear up and occur again within a few days.
These could be signs of a serious condition.