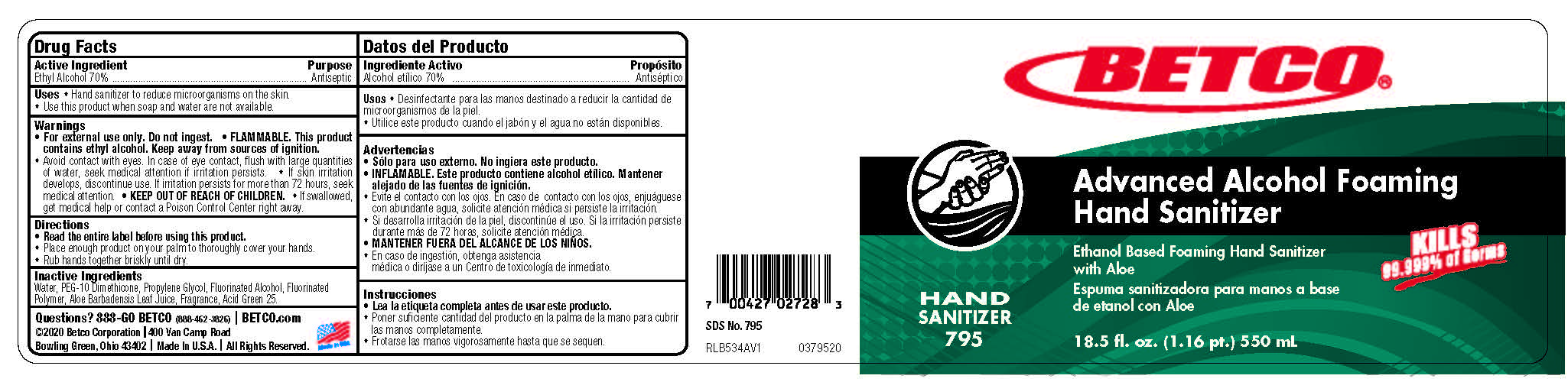
Uses
Uses
- Use hand sanitizer to reduce microorganisms on the skin.
- Use this product when soap and water are not available.
Warnings
Warnings
- For external use only. Do not ingest.
- FLAMMABLE. This product contains ethyl alcohol. Keep away from sources of ignition.
- Avoid contact with eyes. In case of eye contact, flush with large quantities of water, seek medical attention if irritation persists.
- If skin irritation develops, discontinue use. If irritation persists for more than 72 hours, seek medical attention.
- Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away
Directions
Directions
Read the entire label before using this product.
Place enough product on your palm to thoroughly cover your hands.
Rub hands together briskly until dry.